Electrochemically Synthesized Poly(2-Aminobenzenesulphonic Acid) – An Efficient Protection for Carbon Steel Corrosion
Sini Varghese Cheruvathur1, Joby Thomas Kakkassery*2 , Vinod Raphael Palayoor3, Binsi M. Paulson4 and Ragi Kooliyat5
, Vinod Raphael Palayoor3, Binsi M. Paulson4 and Ragi Kooliyat5
1,2,4,5Department of Chemistry, St. Thomas’ College (Autonomous), Thrissur, Kerala, 680001-India.
3Department of Chemistry, Government Engineering College, Thrissur, Kerala, 680009-India.
Corresponding Author E-mail: drjobythomask@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350223
Article Received on : 06-02-2019
Article Accepted on : 25-01-2019
Article Published : 24 Apr 2019
The corrosion protection efficacy of electrochemically synthesized poly(2-aminobenzenesulphonic acid) (P2ABSA) on carbon steel in 1.0 M HCl was investigated by electrochemical impedance spectroscopy, Tafel polarisation, scanning electron microscopy (SEM) and FT-IR spectral studies. The polymeric coating was prepared on the steel surface using cyclic voltammetry. Investigations established that P2ABSA effectively prevent the metal dissolution in HCl medium. Polarisation studies revealed that this polymer hinder both anodic and cathodic process of corrosion appreciably. The structures of the chemically and electrochemically synthesised polymers were compared using IR spectroscopy. Morphology of the steel surface confirmed the intact response of P2ABSA on steel surface treated with HCl.
KEYWORDS:Corrosion; Electrochemical Impedance Spectroscopy; Poly(2-Aminobenzenesulphonic Acid); Tafel Plots,
Download this article as:| Copy the following to cite this article: Cheruvathur S. V, Kakkassery J. T, Palayoor V. R, Paulson B. M, Kooliyat R. Electrochemically Synthesized Poly(2-Aminobenzenesulphonic Acid) – An Efficient Protection for Carbon Steel Corrosion. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Cheruvathur S. V, Kakkassery J. T, Palayoor V. R, Paulson B. M, Kooliyat R. Electrochemically Synthesized Poly(2-Aminobenzenesulphonic Acid) – An Efficient Protection for Carbon Steel Corrosion. Orient J Chem 2019;35(2). Available from: https://bit.ly/2PuwB0b |
Introduction
Protection of steel from the aggressive solutions can be done by methods like addition of corrosion inhibitors to the medium, making a protective coating on the surface etc.1-4 Prevention of corrosion by the use of protecting coatings has been an area of interest for last two decades for corrosion scientists and engineers.5,6 Many articles have been reported on the corrosion protection efficacy of organic coatings on stainless steel, carbon steel and mild steel.7-9 Corrosion protection by intrinsically conducting polymers has got attention now days due to the high corrosion protection efficiency for long time.10,11 Several articles have been published on the corrosion protecting capacity of polyaniline doped with various anions like chloride, sulphate, phosphate etc.12-15 The role of dopants on the conducting polymers in preventing the metal surface from corrosion is also revealed by scientists. Apart from the conventional polymer PANI, some researchers tried to explore the corrosion inhibition ability of substituted polyanilines.16 When compared to the polyaniline coating majority perform bad to protect the metal surface. Conducting polymers protect the metal surface in two ways.17-19 Primarily they act as a passive layer and prevent the contact of the aggressive medium with the metal surface.20 Secondly these polymers create an electronic barrier and hence prevent the oxidation of atoms present on the metal surface.21 Scientists and corrosion engineers are always in search of more durable, efficient and economic protective coatings on metal surface to combat against metal dissolution.22,23 In earlier studies the polyaminobenzene sulphonic acid polymerised electrochemically and properties, electric conductivity values found out.24 The compound amino benzene sulphonic acid is used for coating electrochemically on mutiwalled nanotubes and it is also used for the treatment of emeraldine base.25,26,27 The main objective of the present investigation is that to study the corrosion protection efficacy of electrochemically synthesized poly(2-aminobenzenesulphonic acid) (P2ABSA) coating on carbon steel surface in HCl medium.
Materials and Methods
Chemicals and Steel Specimen
All chemicals (2-aminobenzenesulphonic acid, HCl and ammonium persulphate) were purchased from Merck millipore. Carbon steel specimens of chemical composition C,0.55%; Mn,0.08%; P,0.04%; S,0.012%; Si,0.02% and rest Fe (determined by EDAX method) were cut (15x4cm) and abraded with various grades of SiC paper. These steel strips were washed with soap solution followed by acetone and used for electrochemical coating.
Synthesis of P2ABSA
Electrochemical Polymerisation
Conducting polymeric coating on the steel surface was done using cyclic voltammetry (CV). Three electrode cell assembly was used for voltammetric studies. A 0.2M solution of amine was prepared in 1.0 M HCl. 50 ml of this solution was taken in the electrolytic cell. Inserted carbon steel electrode having 1.0 cm2 exposed area into the solution, which acted as working electrode, and platinum electrode as inert electrode. Reference electrode used was Ag-AgCl electrode. A potential range of +2 to -2 V with a scan rate of 0.1V/sec was used in voltammetric analysis. For ensuring good coating of conducting polymer on the steel surface, CV was done for 10 cycles. After the voltammetric experiment, the working electrode was taken outside, washed with distilled water and dried.
Chemical Polymerisation
A 1.0 M solution of 2-aminobenzenesulphonic acid (100ml) is prepared in 1.0 M HCl. The temperature of the solution was kept between 0-50C. To this solution 100ml 1.0 M solution of ammonium persulphate in 1.0 M HCl was slowly added for 20 minutes. The precipitated product was filtered, washed with acetone and dried at 1000C for 1 h.
Electrochemical Corrosion Studies
Electrochemical corrosion investigations were done on the steel specimen coated with P2ABSA at two different conditions i) the coated steel specimen was kept for 24 h in air and immersed in 1.0 M HCl for attaining open circuit potential (OCP) and the electrochemical tests were conducted. ii) the coated portion of the metal specimen was immersed in 1.0 M HCl (100ml) for 24 h and performed electrochemical studies.
Impedance Measurements
A three electrode cell assembly was made to perform the electrochemical experiments. In this, saturated calomel electrode (SCE) acted as reference electrode, platinum foil (1cm2) acted as inert electrode and the steel specimen coated with the conducting polymer P2ABSA (exposed area 1cm2) as the working electrode. 1.0 M HCl was taken as the aggressive medium for the study.28,29 Before performing EIS analysis it was verified that the OCP had reached to a steady state. A frequency range of 1 KHz to 100 mHz at a scan rate of 1mV/sec was employed for impedance study. EIS analysis was also performed on an uncoated steel specimen treated with 1.0 M HCl for 24 h. Analysis of the Nyquist plots gave the charge transfer resistances from which the corrosion protection efficiency of the polymeric coating was calculated using the following equation.
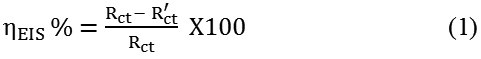
Rct and R’ct represent the charge transfer resistance of coated and uncoated samples respectively.
Tafel Polarization Studies
The coated steel specimens were subjected to Tafel polarization studies in HCl30 for a period of 24 h. As described in EIS studies, three electrode cell assembly was used for polarization investigation. The specimens were scanned between -250 to +250 mV against corrosion potential at a rate of 1mV/s. Analysis of the Tafel plots gave corrosion current densities (Icorr) of the steel specimens.31,32 The corrosion protection efficacy of the polymeric coating was calculated using equation 2.
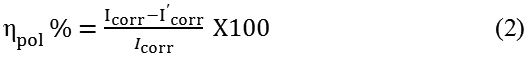
Here Icorr and I’corr represent the corrosion current densities of the uncoated and coated steel specimens respectively.
IR spectral studies
To verify the structure of conducting polymer synthesized electrochemically on the steel surface, FT-IR spectral studies were conducted. The film deposited on the steel surface was carefully removed and subjected to FT-IR spectroscopic analysis using KBr pellet method (Make-Shimadzu, Model-IR Affinity 1 spectrophotometer). This spectrum was compared with the IR spectrum of the polymer synthesized chemically (using ammonium persulphate as oxidant)
Surface morphological studies
To determine the modifications occurred to steel surface coated with P2ABSA, on keeping in HCl for 24 h, scanning electron microscopic studies were performed (JEOL JSM- 6390LV/JED-2300). Micrographs of bare steel surface, uncoated specimen treated with 1.0 M HCl for 24 h etc were also captured and compared.
Results and Discussion
Impedance studies
Analysis of the impedance plots gave parameters like charge transfer resistance (Rct), double layer capacitance (Cdl) and solution resistance (Rs). A simple equivalent circuit (Randles circuit) consist of the above parameters was used for the analysis of Nyquists plot of blank specimen, while the equivalent circuits consisting of Warburg impedance was suitable for the coated specimens33,34 (Figure 1 and 2). The parameters obtained from EIS studies are given in Table 1, sample 1 (uncoated steel specimen immersed in 1.0 M HCl for 24 h), sample 2 (P2ABSA coated steel specimen was kept for 24 h in air and immersed in 1.0 M HCl) and sample 3 (P2ABSA coated steel specimen was immersed in 1.0 M HCl (100ml) for 24 h). From the table it is evident that the charge transfer resistance of steel specimen coated with P2ABSA (sample 2 and 3) was higher than that of the uncoated specimen (sample 1). Sample 2 and 3 displayed very high values of charge transfer resistance indicating that P2ABSA acts as an excellent protection coating on steel surface. Figure 3 represent the Nyquist plots of uncoated and coated steel specimens in 1.0M HCl.
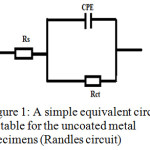 |
Figure 1: A simple equivalent circuit suitable for the uncoated metal specimens (Randles circuit). |
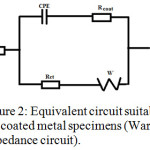 |
Figure 2: Equivalent circuit suitable for the coated metal specimens (Warburg impedance circuit). |
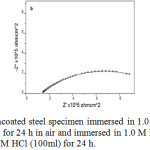 |
Figure 3: Nyquist plots of a) uncoated steel specimen immersed in 1.0 M HCl for 24 h b) P2ABSA coated steel specimen was kept for 24 h in air and immersed in 1.0 M HCl c) The P2ABSA coated steel specimen was immersed in 1.0 M HCl (100ml) for 24 h. |
Table 1: EIS parameters of uncoated and coated steel specimens immersed in 1.0 M HCl for various periods.
|
Sample |
Cdl(µFcm-2) |
Rs(Ωcm2) |
Rct(Ωcm2) |
Rcoat(Ωcm2) |
W(Ωcm2) |
η% |
|
Sample 1 |
114.7 |
9.0 |
149 |
– |
– |
– |
|
Sample 2 |
1.09×10-4 |
10.2 |
6.14×104 |
1.01×106 |
1.09×106 |
99.75 |
|
Sample 3 |
1.27×10-4 |
11.5 |
5.60×104 |
1.42×105 |
2.75×105 |
99.73 |
Tafel Polarisation Studies
Table 2 displays the polarization parameters of uncoated and coated steel specimens with P2ABSA. Similar to the results of impedance studies, the corrosion protection efficacy of the coating was very high on the steel surface in 1.0 M HCl. It can be assumed that the chain of this conducting polymer interact well on the steel surface mainly through co-ordinate bond.35,36 On analysing the Tafel plots, it is evident that slopes of Tafel lines of sample 2 and 3 changed appreciably when compared to that of blank (sample1). Thus from the polarization studies it is revealed that P2ABSA retard the rate of anodic and cathodic process of CS corrosion in 1.0 M HCl considerably.
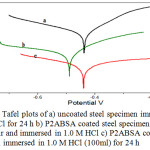 |
Figure 4: Tafel plots of a) uncoated steel specimen immersed in 1.0 M HCl for 24 h b) P2ABSA coated steel specimen, kept for 24 h in air and immersed in 1.0 M HCl c) P2ABSA coated steel specimen immersed in 1.0 M HCl (100ml) for 24 h. |
Table 2: Potentiodynamic polarization parameters of uncoated and coated steel specimen immersed in 1.0 M HCl for various periods.
| Sample |
Icorr (µA/cm2) |
ba (mV/dec’) |
bc (mV/dec’) |
-Ecorr (mV) |
ƞpol % |
|
Sample 1 |
111.4 |
83 |
173 |
439 |
– |
|
Sample 2 |
1.956 |
142 |
328 |
519 |
98.24 |
|
Sample 3 |
0.129 |
424 |
210 |
460 |
99.88 |
Surface morphological studies
To examine the morphology of the surface of uncoated and coated steel specimens, scanning electron microscopic studies were conducted. Figure 5 represent the micrographs of bare steel surface, steel specimen treated with 1.0 M HCl for 24 h (sample 1), steel surface coated with P2ABSA and immersed in 1.0 M HCl for 24 h (sample 2) respectively. The surface morphology of bare and blank specimens was entirely different. The surface of the blank specimen was irregular and rough due to the reaction between surface iron atoms and HCl. Micrograph of the steel specimen coated with P2ABSA was appeared to be smoother than blank specimen (sample 1), indicating than a layer of polymer was strongly bound on the steel surface.
 |
Figure 5: Scanning electron micrographs of a) bare metal surface b) uncoated steel specimen immersed in 1.0 M HCl for 24 h c) P2ABSA coated steel specimen was kept for 24 h in air and immersed in 1.0 M HCl d) P2ABSA coated steel specimen immersed in 1.0 M HCl (100ml) for 24 h. |
IR Spectral Investigations
To verify the structure of P2ABSA deposited on the steel surface, the same was synthesized chemically using ammonium persulphate as oxidant. Chemically and electrochemically synthesized polymers were analysed by FT-IR spectroscopy25 and compared. IR spectra of two polymeric samples are given in Figure 6. A close similarity was observed for the IR spectrum of both samples. The proposed structure of P2ABSA is given in Figure 7. Important frequencies displayed by P2ABSA and their possible assignments are given in Table 3. The difference of the IR frequencies of electrochemically synthesized polymer from the chemically synthesized sample can be attributed to the interaction of P2ABSA chain on Fe atoms on the steel surface.
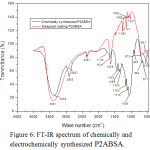 |
Figure 6: FT-IR spectrum of chemically and electrochemically synthesized P2ABSA. |
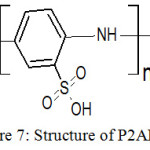 |
Figure 7: Structure of P2ABSA. |
Table 3: Characteristic IR frequencies (cm-1) of chemically and electrochemically synthesized P2ABSA.
| IR frequencies of chemically synthesized P2ABSA(cm-1) | IR frequencies of electrochemically synthesized P2ABSA(cm-1) | Assignment |
|
3348 |
3381 |
N-H stretching |
|
2905 |
2927 |
N-H, O-H stretching |
|
1641 |
1626 |
C=N stretching |
|
1325 |
1262 |
C-C bending |
|
1163 |
1147 |
S-O stretching |
|
1113 |
1082 |
S-O stretching |
|
1059 |
1027 |
S-O asymmetric stretching |
|
897 |
– |
C-H bending (oop)/ring breathing |
Conclusions
Poly(2-aminobenzenesulphonic acid) was coated electrochemically on carbon steel surface. Using IR spectroscopy the structures of P2ABSA synthesized by electrochemical and chemical methods were compared.The corrosion protection behaviour of P2ABSA in 1.0M HCl was evaluated using electrochemical techniques. P2ABSA showed greater than 99% of corrosion protection efficiency on carbon steel surface in 1.0M HCl. According to EIS studies, the charge transfer resistance of coated specimen was very high than that of blank specimen. Considerable lowering of corrosion current densities of the coated samples were noticed in Tafel studies. The potentiodynamic polarisation study revealed that P2ABSA significantly decreased the rate of anodic and cathodic corrosion process of carbon steel in 1.0M HCl. The surface morphology of carbon steel surface (coated and uncoated) was determined by scanning electron microscopy.
Acknowledgements
Authors are grateful to UGC for providing the financial assistance for the research work.
Conflicts of Interests
There is no conflict of interest.
References
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G.; Trivedi, D.C. Prog. Org. Coat. 2005, 53(4), 297-301.
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Electrochim. Acta. 2006, 51(28), 6313-6319.
- Sathiyanarayanan, S.; Jeyaram, R.; Muthukrishnan, S.; Venkatachari, G. J. Electrochem. Soc. 2009, 156(4) 127-134.
- Stanly, K.J.; Geetha, P. Corro. Sci. 2010, 52(1) 224-228.
- Vandana, P. S.; Pradip, P. P. J. Solid State Electrochem. 2013, 17(1), 29-41.
- Priya, A.S.; Muralidharam, V.S.; Subramannia, A. Corros. 2008, 64(6), 541-552.
- Medrano-Vaca, M.; Gonzalez-Rodriguez, J.; Nicho, M.; Casales, M.; Salinas-Bravo, V. Electrochim. Acta. 2008, 53(9), 3500–3507.
- Shokry, H. Chem. Met. Alloys. 2009, 2, 202-210.
- Ohtsuka, T. Int. J. Corros. 2012, 2012, Article ID 915090, 1-7.
- Khan, M. I.; Chaudhry, A. U.; Hashim, S.; Zahoor, M. K.; Iqbal, M. Z. Chem. Eng. Res. Bulletin. 2010, 14(2), 73-86.
- Kenneth, J. C.; Andrew, J. V.; Maocheng, Y.; Thomas, A. S.; Samuel, B.; Victoria, J. G.; Electrochim. Acta. 2011, 56(23), 7796-7804.
- Pawar, P.; Gaikawad, A. B.; Patil, P. P. Sci. Technol. Adv. Mater. 2006, 7(7), 732-744.
- Dai, Y.; Zhu, F.; Zhang, H.; Ma, H.; Wang, W.; Lei. J. Int. J. Electrochem. Sci. 2016, 11, 4084 – 4091.
- Quraishi, M. A. ; Prakash, R., Corros. Sci. 2008, 509(10), 2867-2872.
- Kowalski, D.; Ueda, M.; Ohtsuka, T. Corros. Sci. 2007, 49(8), 3442–3452.
- Nguyen Thi Le, H.; Garcia, B.; Deslouis, C.; Le Xuan, Q. Electrochimi. Acta. 2001, 46(26-27), 4259–4272.
- Chen, Y.; Wang, X. H.; Li, J.; Lu, J. L.; Wang, F. S. Corros. Sci. 2007, 49, 3052-3063.
- Bailong, L.; Zhaohui, Z.; Jiangkai, W.; Shifeng, L. Int. J. Electrochem. Sci. 2017, 12 994 – 1003.
- Cheng, C.; Shihui, Q.; Songlv, Q.; Guoping, Y.; Haichao, Z.; Liping, W. Int. J. Electrochem. Sci. 2017, 12, 3417 – 3431.
- Sathiyanarayanan, S.; Muthukrishnan, S.; Venkatachari, G. Synth. Met. 2006, 156(18-20), 1208–1212.
- Vinod, P.R.; Joby, T.K.; Shaju, K.S.; Aby, P. Res. Chem. Intermed. 2014, 40, 2689-2701.
- Ohtsuka, T.; Iida, M.; Ueda, M. J. Solid State Electrochem. 2006, 10(9), 714–720.
- Romeiro, A.; Gouveia-Caridade, C.; Brett, C.M.A. Corros. Sci. 2011, 53, 3970-3977.
- Kitani, A.; Satoguchi, K.; Tang, H-Q.; Sasaki, K. Synthetic metals. 1995, 69, 129-130
- Lei, Z.; Zhige, S.; Qiuhua, L. J. Solid State Electrochem. 2011, 15, 801–809.
- Yue, J.; Gordan, G.; Epstein, A.J. polymer. 1992, 33, 4410.
- Kilmartin, P.A.; Wright, G.A. Synth.Metals. 1997, 88,153.
- Shaju, K.S.; Joby, T.K.; Vinod. P.R. Oriental.J. Chem. 2014, 30(2), 807-813.
- Wang, H.L.; Hong-Bo, F.; Jia-Shen, Z. Mater. Chem. Phys. 2003, 77(3), 655-661.
- Lowmunkhong, P.; Ungthararak, D.; Sutthivaiyakit, P. Corros. Sci. 2010, 52(1), 30-36.
- Soltani, N.; Behpour, M.; Ghoreishi, S. M.; Naeimi, H. Corros. Sci., 2010,52 (4), 1351-1361.
- Sabirneeza, A.A.F.; Subhashini, S. J. Appl. Polym. Sci. 2013, 127, 3084-3092.
- Hamdy, H. H.; Essam, A.; Mohammed, A. A. Electrochim. Acta. 2007, 52(22), 6359-6366.
- Nimmy, K.; Joby, T. K; Vinod P. R.; Sini, C.V. Current Chemistry Letters 2017, 6, 177–186.
- Ferreira, E.S.; Giacomelli, C.; Giacomelli, F.C.; Spinelli, A. Mater. Chem. Phys. 2004, 83(1), 129–134.
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Electrochim. Acta. 2005, 50, 3446-3452.

This work is licensed under a Creative Commons Attribution 4.0 International License.









