Electrochemical Data on the Effect of Extreme Temperatures on 2101 Duplex Steel Corrosion Performance in Chloride-Sulphate Environment
Department of Mechanical Engineering, Covenant University, Ota, Ogun State, Nigeria.
Corresponding Author E-mail: tolu.loto@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350204
Article Received on : 09-11-2018
Article Accepted on : 12-03-2019
Article Published : 08 Apr 2019
The detrimental outcome of heat treatment operation at 1200oC on 2101 duplex stainless steel was studied in 2 M H2SO4/0%-1.75% NaCl by potentiodynamic polarization, open circuit potential measurement and optical microscopy analysis. Result reveal the processes heightened the corrosion vulnerability of the duplex alloy with maximum corrosion rate result of 11.3 mm/y at 0.5% NaCl for the annealed steel (A2101ST), followed by quenched steel (Q2101ST) with corrosion rate value of 9.03 mm/y. These values were significantly lower than the peak corrosion rate of the as received steel (ASR2101ST) at 5.785 mm/y. The corrosion rate of all the duplex steels generally decreased beyond 0.5% NaCl. ASR2101ST sustained it passivation characteristics till 1.75% NaCl due to the resilience of its oxide protective film while the passive film of Q2101ST and A2101ST were completely destroyed due to alteration in the metallurgical configuration of the heat treated duplex steels. While the surface of ASR2101ST mildly deteriorated, severe intergranular and pitting corrosion was observed on Q2101ST and the surface of A2101ST was marginally pitted in the presence of SO42- ions. In Cl-/SO4 ion solution ASR2101ST showed an etched morphology with ferrite and austenite phases appearing in addition to superficial pitting. Q2101ST and A2101ST were severely deteriorated in the solution.
KEYWORDS:Corrosion; Microstructure; Pitting; Steel
Download this article as:| Copy the following to cite this article: Loto R. T. Electrochemical Data on the Effect of Extreme Temperatures on 2101 Duplex Steel Corrosion Performance in Chloride-Sulphate Environment. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Loto R. T. Electrochemical Data on the Effect of Extreme Temperatures on 2101 Duplex Steel Corrosion Performance in Chloride-Sulphate Environment. Orient J Chem 2019;35(2). Available from: https://bit.ly/2KelyZZ |
Introduction
Stainless steels are widely applied across all industries considering their excellent physical, mechanical, economical and corrosion resistant properties.1 Duplex stainless steel combines their low nickel content with its high mechanical strength making them a cost effective counterpart to austenitic stainless steel grades. They consist of a ferrite and austenite phase microstructure coexisting together as a result it embodies the characteristics of both ferrite having high strength and durability, and the austenite which is known to have excellent corrosion resistance.2-4 The dual nature of duplex steels enables its versatile application as material of construction for pipeline, pressure vessels and storage tanks, in sea water, well design and in environments where corrosion and wear resistance are high priorities.5-9 Their dual-phase microstructure is highly dependent on their nominal (wt. %) composition and heat treatment history. Heat treatment enables the removal or dissolution of intermetallic phases, micro segregations and to alleviate the effect of residual thermal stresses formed during production operation. Heat treatment processes have mixed effect on duplex stainless steel with respect to temperature range, duration and cooling rates. Though the processes are commonly associated with enhancing strength properties ferrous alloys, it is also utilized in altering manufacturability objectives to improve ductility, machining and formability after plastic deformation processes. Preceding research confirms that quenching heat treatment enhances the corrosion protection of 420 martensitic steel by 60%. However, there was no significant improvement in the corrosion resistance of 316 austenitic steel.10 Application of heat has significantly influenced the corrosion resistance of stainless steels.11,12 The presence of alloying elements such as Cr, Ni and Mo impart excellent corrosion resistant properties on duplex steel nevertheless when subject to extreme heat treatment processes, several harmful precipitates such as carbides, nitrides and intermetallic phase’s forms resulting in weak resistance to intergranular crack and pit formation on the steel.13,14 This research aims to assess and document the effect of extreme temperatures on the corrosion resistance of 2101 duplex stainless steel in chloride sulphate environment.
Experimental Methods
2101 duplex stainless steel (2101ST) obtained commercially is the subject steel alloy under study. The nominal wt. % composition of the steel specimen was determined with Phenom World scanning electron microscope at the Materials Characterization Laboratory, Department of Mechanical Engineering, Covenant, Ogun State, Nigeria. The composition results are displayed in Table 1. The duplex alloys were machined to dimensions with visible surface area of 1.27 cm2. Some of the duplex alloy samples underwent quenching (Q2101ST) and annealing (A2101ST) heat treatment process inside a muffle furnace at a temperature of 1200°C and sustaining them at the particular temperature for 30 minutes. A2101ST was subsequently cooled naturally in air. Q2101ST was promptly cooled in deionized H2O to produce the desired metallurgical configuration. The temperature was kept generally constant with the aid of a regulator having an accuracy ± 10°C and connected to a thermocouple (K-Type). They as received (A2101ST), quenched and annealed duplex samples were subsequently prepared through metallographic technique. Emery grinding papers with grits of 60, 120, 220, 320, 600, 800 and 1000 was used to smoothen the specimens before polishing with diamond liquid paste to 6 µm. The steel specimens where subsequently washed deionized H2O and propanone for electrochemical analysis. Analar grade NaCl purchased from Titan Biotech, India was formulated in concentrations of 0%, 0.25%, 0.5%, 0.75%, 1%, 1.25%, 1.5%, and 1.75% in 200 mL of 2 M H2SO4 solution, formulated from analar grade H2SO4 acid (98%) with deionized H2O.
Table 1: % Nominal Composition of 2101ST.
| Element denotation | Mo | Si | Ni | Cr | Mn | P | N | C | Fe |
| % Composition 2101ST | 0.4 | 1 | 1.8 | 22.8 | 4 | 0.04 | 0.2 | 0.03 | 69.7 |
Potentiodynamic polarization tests was performed on ASR2101ST, Q2101ST and A2101ST at 37°C laboratory temperature with the aid of a tenary electrode system make-up of platinum counter electrodes, Ag/AgCl reference electrodes and acrylic mounted 2101ST working electrodes. The electrodes were placed within a transparent container, containing 200 mL of the 2M H2SO4 solution at the earlier stated NaCl concentrations. The system was connected to Digi- Ivy 2311 potentiostat. Electrochemical polarization plots were constructed at scan rate of 0.0015V/s from -1.5V and +1V preset potentials. The corrosion current, CI (A) corrosion current density, Cd (A/cm2) and corrosion potential, Cp (V) values were determined from the plots by Tafel extrapolation. Other parameters obtained are cathodic Tafel slope, Bc (V/dec), anodic Tafel slope, Ba (V/dec) and polarization resistance Rp (Ω). Corrosion rate, CR (mm/y) and inhibition efficiency, η (%) were calculated from the following mathematical relationship;
![]()
D represents the steel density in (g/cm3); Eq is the duplex steel equivalent weight (g). 0.00327 is a corrosion rate constant. Open circuit potential measurement (OCP) was conducted at 0.05 V/s step potential for 2000s to study the thermodynamic tendency of the duplex steel to corrode at rest potentials. Comparative morphological representations of the duplex alloys surface was evaluated with Omax trinocular metallurgical microscope.
Result and Discussion
Potentiodynamic Polarization Test
The corrosion polarization behavior of ASR2101ST, Q2101ST and A2101ST duplex alloy specimens are shown on the electrochemical plots depicted in Figs. 1-3. Results of the polarization test are shown in Table 2. ASR2101ST generally had the lowest corrosion rate coupled with a proportionate value of corrosion current density. Its corrosion rate values increased with rise in chloride concentration and peaked at 0.5% NaCl, thereafter a progressive decrease was observed till 1.75%. The same phenomenon occurred for Q2010ST and A2101ST at relatively higher corrosion rates; however A2101ST had the highest corrosion rate values signifying low corrosion resistance due to a number of factors such as degradation resulting from precipitation due to depletion of corrosion-resisting elements (Cr, Ni, Mo etc.) within the alloy substrate and intermetallic rearrangements. The corrosion behavior 2101ST duplex steel is subject to the state of its microstructural properties which undergoes significant changes due to phase transformations during heat treatment. Observation of the corrosion potentials of ASR2101ST and Q2101ST showed active passive behavior between anodic and cathodic values as a result of the counterbalancing action of the electrochemical reaction processes occurring on the alloy surface. The potential values for A2101ST appear to be comparatively more electronegative. This is associated with the weakening of the passive protective film and localized corrosion deterioration on the metal alloy surface, hence its relatively higher corrosion rate values.
Observation of the anodic portion of the electrochemical plots of the duplex alloy specimens (Figs. 1-3) before passivation shows a continuous increase in the metastable pitting region with respect to Cl– ion concentration. This is due to initiation of unstable transient corrosion pits. Pit growth during this period is subject to diffusion of metallic cations from the initiated corrosion pit. The metastable pitting current decreased before the formation of the protective oxide film to a constant passivity current density and achieving relative stability. Extended increase in the anodic portion of all the polarization plots was observed beyond 0.5% NaCl concentration (0.75%-1.25% NaCl) as a result of increased oxidation reactions of the duplex alloy due to higher Cl− ion concentration which is responsible for higher metastable pitting nucleation rate. This mechanism reduced the passivation range of the alloys, hence their corrosion resistance properties before collapse at the transpassive region of the plot. At 1.25% NaCl, Q2101ST and A2101ST had significantly reduced passivation range due to active corrosion reactions before passivation when compared to ASR2101ST. The decrease in passivation range of the two aforementioned duplex alloys decreased further at 1.5% NaCl due to delayed passivation of the alloys, whereas ASR2101ST continued to passivate at lower corrosion potentials. At 1.75% NaCl concentration, Q2101ST and A2101ST duplex alloys where unable to passivate throughout the scanned potentials due to destruction of their passive film at the stated chloride concentration. However ASR2101 still passivated at higher corrosion potentials (-0.94V) due to the debilitating action of the chloride ions. Changes in Cl- ion concentration had minimal effect on the pitting potential of the duplex alloys; however a significant increase in pitting corrosion current occurred before breakdown of the alloys.
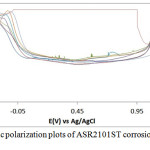 |
Figure 1: Potentiodynamic polarization plots of ASR2101ST corrosion in 2M H2SO4/0% – 1.25% NaCl solution. |
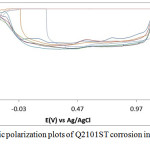 |
Figure 2: Potentiodynamic polarization plots of Q2101ST corrosion in 2M H2SO4/0% – 1.25% NaCl solution. |
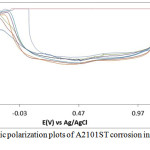 |
Figure 3: Potentiodynamic polarization plots of A2101ST corrosion in 2M H2SO4/0% – 1.25% NaCl solution. |
Table 2: Polarization data on ASR2101ST, Q2101ST and A2101ST corrosion in 2M H2SO4/0% – 1.25% NaCl solution.
|
ASR2101ST |
||||||||
| Sample | NaCl Conc. (%) | CR (mm/y) | CI (A) | Cd (A/cm2) | CP (V) | Rp (Ω) | Bc (V/dec) |
Ba (V/dec) |
| A | 0 | 1.298 | 1.53E-04 | 1.21E-04 | -0.327 | 167.8 | -11.51 | 13.61 |
| B | 0.25 | 3.637 | 4.29E-04 | 3.38E-04 | -0.315 | 59.85 | -8.777 | 4.114 |
| C | 0.5 | 5.785 | 6.83E-04 | 5.38E-04 | -0.324 | 32.82 | -8.829 | 2.468 |
| D | 0.75 | 4.337 | 5.12E-04 | 4.03E-04 | -0.368 | 50.18 | -9.227 | 13.46 |
| E | 1 | 4.315 | 5.09E-04 | 4.01E-04 | -0.334 | 50.44 | -9.022 | 10.13 |
| F | 1.25 | 3.309 | 3.91E-04 | 3.08E-04 | -0.341 | 64.2 | -11.21 | 11.06 |
| G | 1.5 | 2.546 | 3.01E-04 | 2.37E-04 | -0.307 | 76.56 | -10.919 | 9.16 |
| H | 1.75 | 3.27 | 3.86E-04 | 3.04E-04 | -0.253 | 66.56 | -9.55 | 11.03 |
| Q2101ST | ||||||||
| Sample | NaCl Conc. (%) | CR (mm/y) | CI (A) | Cd (A/cm2) | CP (V) | Rp (Ω) | Bc (V/dec) | Ba (V/dec) |
| A | 0 | 2.919 | 3.45E-04 | 2.71E-04 | -0.321 | 74.56 | -9.428 | 9.818 |
| B | 0.25 | 3.637 | 4.29E-04 | 3.38E-04 | -0.327 | 53.8 | -8.252 | 8.949 |
| C | 0.5 | 9.03 | 1.07E-03 | 8.39E-04 | -0.324 | 24.11 | -8.588 | 2.191 |
| D | 0.75 | 4.441 | 5.24E-04 | 4.13E-04 | -0.341 | 49.01 | -9.969 | 14.54 |
| E | 1 | 4.465 | 5.27E-04 | 4.15E-04 | -0.341 | 39.71 | -9.613 | 12.9 |
| F | 1.25 | 3.202 | 3.78E-04 | 2.98E-04 | -0.32 | 67.98 | -10.23 | 12.47 |
| G | 1.5 | 3.287 | 3.88E-04 | 3.06E-04 | -0.245 | 61.34 | -7.441 | 9.145 |
| H | 1.75 | 3.53 | 4.17E-04 | 3.28E-04 | -0.261 | 54.65 | -9.241 | 13.19 |
| A2101ST | ||||||||
| Sample | NaCl Conc. (%) | CR (mm/y) | CI (A) | Cd (A/cm2) | CP (V) | Rp (Ω) | Bc (V/dec) | Ba (V/dec) |
| A | 0 | 2.898 | 3.42E-04 | 2.69E-04 | -0.273 | 75.11 | -7.921 | 2.729 |
| B | 0.25 | 5.156 | 6.09E-04 | 4.79E-04 | -0.29 | 42.21 | -8.187 | 2.815 |
| C | 0.5 | 11.3 | 1.33E-03 | 1.05E-03 | -0.284 | 19.26 | -8.703 | -0.154 |
| D | 0.75 | 9.114 | 1.08E-03 | 8.47E-04 | -0.307 | 38.02 | -9.125 | 12.93 |
| E | 1 | 8.818 | 1.04E-03 | 8.20E-04 | -0.296 | 42.53 | -8.825 | 5.787 |
| F | 1.25 | 7.702 | 9.09E-04 | 7.16E-04 | -0.34 | 52.79 | -10.14 | 11.16 |
| F | 1.25 | 7.702 | 9.09E-04 | 7.16E-04 | -0.34 | 52.79 | -10.14 | 11.16 |
| G | 1.5 | 6.86 | 8.10E-04 | 6.38E-04 | -0.254 | 66.49 | -8.472 | 8.966 |
| H | 1.75 | 5.053 | 5.97E-04 | 4.70E-04 | -0.266 | 83.01 | -9.144 | 6.968 |
Optical Microscopy Analysis
Morphological illustrations (mag. x40 and x100) of ASR2101ST, Q2101ST and A2101ST duplex alloys before corrosion, and after corrosion from 2M H2SO4 and 2 M H2SO4/1.75% NaCl are shown from Fig. 4(a) to 6(c). The optical images before corrosion [Fig. 4(a) – (c)] are generally similar, though serrated edges are more visible on the quenched and annealed steel. The optical image of ASR2101ST [Fig. 5(a)] revealed very few corrosion pits due to the electrochemical reaction induced by SO42- ions without chlorides; however the extent of morphological damage is much more visible on Q210ST alloy [Fig. 5(b)] where extensive intergranular cracks and micro and macro corrosion pits in the region of the cracks due to the combined electrochemical action of SO42- and Cl- ions. The annealed alloy [Fig. 5(c)] did not experience intergranular corrosion but well defined corrosion pits can be observed. These observations are the product of different phase transformations in the metallurgical structure of 2101ST resulting from the heat treatment processes. Results from electrochemical test showed that ASR2101ST had the lowest corrosion rate however it appears A2101ST was less susceptible to localized corrosion reactions. The morphological damage of ASR2101ST [Fig. 6(a)] is quite extensive compared to Fig. 5(a); intergranular cracks and propagated corrosion pits are clearly visible in addition to the ferrite and austenite phases which can be seen on the alloy. Compared to Fig. 6(b) and (c) the morphological deterioration in Fig. 6(a) seems quite superficial as the degree of localized corrosion deteriorations on the quenched and annealed duplex alloy are quite deeper signifying the detrimental effect of heat treatment on 2101ST.
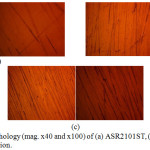 |
Figure 4: Surface morphology (mag. x40 and x100) of (a) ASR2101ST, (b) Q2101ST and (c) A2101ST before corrosion. |
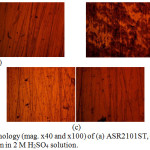 |
Figure 5: Surface morphology (mag. x40 and x100) of (a) ASR2101ST, (b) Q2101ST and (c) A2101ST after corrosion in 2 M H2SO4 solution. |
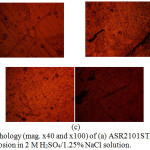 |
Figure 6: Surface morphology (mag. x40 and x100) of (a) ASR2101ST, (b) Q2101ST and (c) A2101ST after corrosion in 2 M H2SO4/1.25% NaCl solution. |
Open Circuit Potential Measurement (OCP)
The thermodynamic stability of ASR2101ST, Q2101ST and A2101ST duplex alloys in 2 M H2SO4, 2 M H2SO4/0.25% NaCl and 2 M H2SO4/1.75% NaCl are shown from Fig. 7(a) to 7(c). Plot of OCP values versus exposure time for the duplex alloys as shown in the figures indicates that none of the alloys with the exception of Q2101ST in 2 M H2SO4 [Fig. 7(a)] achieved relative stability for the 2000 s of exposure time. Q2101ST shifted to -0.300 V at 100 s due to instantaneous formation of the passive film, thereafter in decline progressively to -0.305 V at 600 s due to collapse of the passive film, probably as a result of localized corrosion reactions in the presence of SO42- ions. Beyond 600 s, the OCP value of Q2101ST was relatively stable till 2000 s ASR2101ST and A2101ST in Fig. 7(a) experienced brief decrease in OCP for the first 200 s and 100 s (-0.308 V and -0.281 V) due to active corrosion reactions on the alloy surface, after which a progressive increase in OCP was observed due to formation of the passive protective film on the alloys surfaces. Similar phenomenon was observed for ASR2101ST, Q2101ST and A2101ST in Fig. 7(b), however A2101ST after declining to -0.278 V (100 s) was relatively stable till 1050 s (-0.277 V) due to active-passive corrosion behavior resulting instantaneous breakdown and repassivation of the oxide film occurring on the alloy surface; after which the OCP value progressively increased to 2000 s. It must be noted that at rest potential A2101ST is the most corrosion resistant duplex alloy despite the contrary from potentiodynamic polarization and optical microscopy where ASR2101ST was determined to be the most susceptible to corrosion. The significant variation in OCP between A2101ST and other duplex alloys is due to the strength of its passive oxide layer which tends to be more resistant to corrosion compared to ASR2101ST and A2101ST. In 2 M H2SO4/1.75% NaCl [Fig. 7(c)], the OCP values of A2101ST was the most electronegative till 1850 s (-0.251 V) of exposure due to comparatively weaker corrosion resistance compared to ASR2101ST and Q2101ST, however the relative increase in OCP values shows the oxide film on the three duplex alloy specimens is rapidly forming in the presence of (1.75% NaCl). Observation of the OCP plots in Fig 7(c) also shows the relatively weak corrosion resistance of Q2101ST. At 250 s (-0.276) the rate of increase in OCP values begins to decrease due to earlier formation of its oxide film which indicates the film is quite thinner compared to ASR2101ST and A2101ST. It eventually overlaps with A2101ST at 1850 s.
 |
Figure 7: Plot of OCP versus exposure time for ASR2101ST, Q2101ST and A2101ST (a) 2 M H2SO4, (b) 2 M H2SO4/0.25% NaCl and (c) 2 M H2SO4/1.75% NaCl. |
Conclusion
Quenching and annealing heat treatment processes at extreme temperature of 1200°C deteriorated the corrosion resistance of 2101 duplex stainless in 2 M H2SO4/0% – 1.75% NaCl by 36% and 48%. While the as received duplex steel retained its corrosion resistance and passivation behaviour at all chloride concentrations studied, the quenched and annealed duplex steels lost their passivity. Optical microscopy images showed severely deteriorated morphology with visible intergranular cracks and corrosion pits.
Acknowledgements
The author recognizes the immeasurable support by Covenant University Ota, Ogun State, Nigeria in the sponsorship and provision of research facilities for this project.
References
- Kai Wang, C.; Sie Chin, T. Materials. 2014, 7(7), 5268–5304 (2014). doi: 10.3390/ma7075268.
- Charles, J. Weld. World, 1995, 36, 43-54.
- Park, C.J.; Kwon, H.S. Corros. Sci. 2002, 44(12), 2817-2830.
- Esteves, L.; Cardoso, M.; Lins, V.F.C. Mats. Res. 2018, 12(1). doi.org/10.1590/1980-5373- mr-2017-0148.
- Bastos, I.N.; Tavares, S.S.M.; Dalarda, F.; Nogueira, R.P. Scr. Mater. 2007, 57(10), 913– 916.
- Deng, B.; Jiang, Y.; Gong, J.; Zhong, C.; Gao, J.; Li, J. Electrochim. Acta. 2008, 53(16), 5220–5225.
- Igual Munoz, A.; Garcia Anton, J.; Guinon, J.L.; Perez Herranz, V. Corros. Sci. 2006, 48(12), 4127–4151.
- Nascimento, A.M do.; Ierardi, M.C.F.; Kina, A.Y.; Tavares, S.S.M. Mater. Charact. 2008, 59(12), 1736–1740.
- Vesna, A., Ivan, S.; Bruno, Z.; Franjo, I. Int. J. Elect. Sci. 2013, 8, 12476 –12486.
- Loto, R.T.; Aiguwurhuo, O.; Ukene, E. Rev. Téc. Ing. Univ. Zulia. 2016, 39(7), 35-40.
- Loto, R.T.; Loto, C.A. Corrosion behaviour of S43035 ferritic stainless steel in hot sulphate/chloride solution. J. Mater. Res. Technol. 2018, 7(3), 231-239.
- Loto, R.T. Effect of elevated temperature on the corrosion polarization of NO7718 and NO7208 nickel alloys in hot acid chloride solution. J. Bio Tribo. Corr. 2018, 4:71. https://doi.org/10.1007/s40735-018-0190-8.
- Alvarez-Armas, I.; Degallaix-Moreuil, S. Duplex Stainless Steels, Wiley; Hoboken, NJ, USA, 2009. doi.org/10.1002/9781118557990.fmatter.
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Mater. Sci. Eng. R Rep. 2009, 65 (4-6), 39–104. doi: 10.1016/j.mser.2009.03.001.

This work is licensed under a Creative Commons Attribution 4.0 International License.










