“Design and Synthesis of Some Cyclic Carbohydrates with Norfloxacin as Surface Moiety. A Study of Sustained Relax of Drugs”.
J. Dhevaraj, S. Vembu, S. Pazhamalai* and M. Gopalakrishnan
and M. Gopalakrishnan
Department of Chemistry, Annamalai University, Annamalai nagar 608002, Tamil Nadu, India.
Corresponding Author E-mail: sripazhamalai@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350211
Article Received on : 15-07-2018
Article Accepted on : 02-03-2019
Article Published : 16 Apr 2019
Biocompatible and biodegradable sustained drug delivery system has been constructed from reaction between norfloxacin and cyclodextrin through secondary amine of piperazine ring and hydroxyl group of the carbohydrate. Covalent bond polymeric structure is designed by the help of chloroacetyl chloride, target dendrimer formed by removing two hydrochloride molecules. The development of cyclodextrin core drug delivery system with twenty one norfloxacin surface moiety has been synthesized by only two steps. The synthesized polymeric structure was thoroughly studied by NMR, FT-IR, MALDI and UV- spectrometry. Sustained release assessment of synthetic polymer studied through different buffer solution by UV spectrometry and norfloxacin releases rate of synthetic polymer was controlled by the concentration and the experimental medium. The microbial assessments through kinetic studies by using Escherichia coli also reveal that the norfloxacin released possesses potential antimicrobial activity. Antibacterial activity of synthesized drug delivery system has been investigated with gram-negative and gram-positive species like Escherichia coli (mtcc 443), bacillus subtilis (mtcc 2063), pseudomonas (mtcc 741), staphylococcus (mtcc 737) and proteus mirabilis (mtcc 425). The hydrophobic and hydrophilic balance and the repeat drug unit of this synthesized system are responsible for effective antibacterial activity. The minimum inhibitor concentration values of this system are very small to 100 µg/mL-1, synthesized compound shown five times improved activity against organism on comparism with standard drug. The in-vitro release of norfloxacin from obtained dendrimer was investigated.
KEYWORDS:Chloroacetyl Chloride; Cyclodextrin, Dimethyl Sulfoxide; Norfloxacin; Tetrahydrofuran; Triethylamine
Download this article as:| Copy the following to cite this article: Dhevaraj J, Vembu S, Pazhamalai S, Gopalakrishnan M. “Design and Synthesis of Some Cyclic Carbohydrates with Norfloxacin as Surface Moiety. A Study of Sustained Relax of Drugs”. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Dhevaraj J, Vembu S, Pazhamalai S, Gopalakrishnan M. “Design and Synthesis of Some Cyclic Carbohydrates with Norfloxacin as Surface Moiety. A Study of Sustained Relax of Drugs”. Orient J Chem 2019;35(2). Available from: https://bit.ly/2PbMSam |
Introduction
β-cyclodextrin and its derivatives are generally used in pharmacology as containers for some medicines owing to their unique property of encapsulating different hydrophobic compounds by formation of the inclusion compounds of guest-host type.1 Such encapsulation complex the included medicine from bio-decomposition, contributes to increase in its solubility and that is of considerable importance to effective and selective delivery of the medicine to the important place for a short time. However, in the recent past much attention is paid to investigate the covalent binding or conjugation of a medicine to cyclodextrin that allows preparing new medicines with more prolonged and goal directed action.2
Cyclodextrin- Assemblies
The three member family of cyclic oligosaccharides called cyclodextrins (CDs) (α-, β-, and γ-CDs), are linked by α-1,4-glucopyranose units (Figure 1). Hydrophobic inner cavity and hydrophilic outer surface is important feature in cyclodextrin, the shape resembling like a bridged cone. Cyclodextrin not only used to excellent receptor for molecular recognition, it is extensively used to fashionable building blocks for supramolecular assembly. The exceptional applications of cyclodextrins are due to (i) their soluble ability in aqueous medium (ii) it’s action as receptor molecule to a broad variety of guest molecules and (iii) their well-defined molecular structure.3-7,8-12
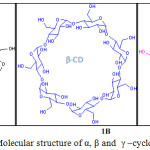 |
Figure 1: Molecular structure of α, β and γ –cyclodextrin. |
Norfloxacin
Norfloxacin, a fluoroquinoline oral anti-biotic is active against pseudomonas aeruginosa and penicillin resistant strain of nisseria gonorrhoea. The drug is less active against gram-positive cocci. Norfloxacin is inhibiting the subunits of DNA gyrase due to its bactericidal effect, which is essential for DNA replication of bacteria.
Based on the formerly mentioned findings, the important aim of this research is to design sustain drug delivery system with biodegradable artificial polymer with well define shape, monomeric properties and analogues to the protein have been known to literature as dendrimer. Theses synthesized drug delivery system almost in a spherical shape, the diameters normally from 2 to 10nm with excellent novel and sustain drug delivery application system13,14 derivatives of norfloxacin for the purpose of improving the antimicrobial performance of norfloxacin and the control drug delivery system construction through covalent bond interaction. β-cyclodextrin is versatile platform to form spherical shape drug released system are architecture through covalent bond interaction between cyclodextrin and norfloxacin. The cells of v.parahaemolyticus have an energy dependent efflux system for drug molecule; they cloned a gene for a putative norfloxacin efflux protein from the chromosomal DNA of v.parahaemolyticus with an Escherichia coli mutant lacking the major multidrug efflux system.
Cell based assay methods are important part of the synthetic organic field to evaluate the ability of biological activity. Antibacterial activity of synthesized compound tested against different bacteria or cancer cell and the synthesized compounds are inhibited their growth against sustain drug delivery. In this study, we examined the norfloxacin drug release of synthesized dendrimer by using Staphylococcus aureus (mtcc 737, Gram positive) and Escherichia coli (mtcc-443, gram negative) microorganism.
Results and Discussion
Chemistry
The twenty one number of hydroxyl group of narrower end primary (bonded to the C6 atoms) and broadest end secondary (bonded to the C2 and C3 atoms) hydroxyl groups in β-cyclodextrin core play an important role to design this drug delivery system structure. The delivery system with twenty one numbers of norfloxacin molecules in the place of hydroxyl groups as a surface moiety has been synthesized in two steps, in order to improve the activity and its sustained delivery of dendrimeric drug. In the first step, chloroacetyl β-cyclodextrin is synthesized by the reaction in between chloroacetyl chloride and β-cyclodextrin using N-Ethyldiisopropylamine as a catalyst in tetrahydrofuran under refluxing condition. In this reaction, twenty one chloroacetyl chloride moieties are attached to β-cyclodextrin hydroxyl group. Further the norfloxacin and chloroacetyl β-cyclodextrin is mixed in the ratio of 1: 21 to react under the above reaction condition gives the target molecule. In this, exactly twenty one piperazine of norfloxacin molecule react with twenty one chlorine groups of chloroacetyl β-cyclodextrin to form biodegradable norfloxacin dendrimer as the synthetic route is illustrated in Scheme 1.
Chloroacetyl norfloxacin was synthesized by mixing 1:21 ratio of norfloxacin and chloroacetyl chloride (NFLX, donated by Cipla Ltd, Bombay) was dissolved in 25 ml tetrahydrofuran and then added N-Ethyldiisopropylamine. The above mixture was refluxed to 8 hrs, after completion of the reaction mixture was poured into ice water and the residue was extracted with ethyl acetate (3 x 50 ml). The combined ethyl acetate extracts were washed with water, Chloroacetyl norfloxacin separated as yellow colour fraction under reduced pressure. In the next step, 1:21 ratio of chloroacetyl β-cyclodextrin and norfloxacin react under the reaction condition to give the target molecule with good yield. The β-cyclodextrin suitable core moiety to construction of drug delivery system, it directly connected with twenty one chloroacetyl chloride groups, at the second stage twenty one norfloxacin moiety directly connected with chloroacetyl β-cd through covalent bond. Twenty one hydroxy groups are reacting with equal number of chloroacetyl chloride to form chloroacetyl β-cd by loss of HCl moleculs in the presence of base molecule. Twenty one piperazine of norfloxacin molecule replace with twenty one chlorine groups of chloroacetyl β-cyclodextrin to form norfloxacin moiety contain biodegradable system and the synthetic route of the norfloxacin drug delivery system is as illustrated in the Schemes 1.
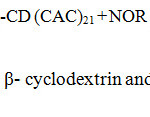 |
Scheme 1: Synthesis of chloroacetyl β- cyclodextrin and Norfloxacin dendrimer with β- cyclodextrin. |
Experimental Section
The synthesized polymeric structure was involved systematically characterization through NMR, FT-IR, MALDI-TOF and UV- spectrometry. The vibrational spectra of the dendrimer were recorded in Agilent carry 630 spectrometry with a globar source, Ge/KBr beam splitter and a MCT detector. Melting points were observed by the open capillary method and are uncorrected. 1H and 13C nuclear magnetic resonance (NMR) (400 MHz; CDCl3 or DMSO) spectra were recorded at 293 K on BRUKER AVANCE III AMX-400 spectrometry operating frequencies of 400 MHz for proton and 100 MHz for carbon using CDCl3 or DMSO as solvent.. 5 mg test sample was dissolved in 0.5 ml of solvent (CDCl3, DMSO) to test solution preparation. All NMR Measurements were made on 10 cm NMR tubes and all the chemical shifts values are reported in parts per million scales (δ-scale) downfield from tetramethylsilane. MALDI-TOF mass spectrum was recorded on AB SCIEX TOF/TOF 5800. Sinapic acid was used as a matrix for all experiment. The matrix-samble ratio maintained at 100:1 throughought the experiments.
Synthesis of Chloroacetyl β-cyclodextrin and Its Spectral Studies
The newly purchased chemicals are used as it is to synthesis the target molecule. One millimole of β-cyclodextrin dissolved in tetrahydrofurane and adding twenty one millimole of chloroacetyl chloride in presence of triethyl amine. The reaction mixture was refluxed for 4-6 hrs, after completion of reaction; the reaction mixture was cooled to room temperature and poured into ice water. The final product was extracted with ethyl acetate and the chloroacetyl β-cyclodextrin fraction separated by reduced pressure method. Obtained fraction was used to different characterization and further stages. The IR spectrum of compound 2 in (KBr): acetyl ketone appeard at 1756 cm-1 (γC=O) and 2961cm-1 for aliphatic γC-H. 1H NMR (CDCl3, TMS, 27°C, 400 MHz) δ 3.57 (d, second carbon CH proton), δ 3.603 (d, fourth carbon proton of glucopyranose ring). δ 4.1 (fifth carbon proton of glucopyranose ring ), δ 4.25 (third position carbon proton of glucopyranose ring) 4.72 (sixth position proton of glucopyranose) and δ 5.57 (-CH2 protonsof acetyl chloride ).13C NMR (CDCl3, TMS, 27°C, 400MHz) δ62.12 (sixth position carbons of glucopyranose unit ), δ29.59 (second position carbon). δ 44.90 (third position carbon), δ 66.10 (first carbon signal of glucopyranose ring), δ 42.30 (fifth carbon in glucose ring), δ 32.84(fourth carbon of clucopyranose residue), δ 65.48 (acetyl chloride carbon) and δ 170.81 (acetyl chloride C=O).
Synthesis of Norfloxacin Based β-cyclodextrin Core Drug Delivery System (Compound-3) and its Spectral Studies
One millimole of chloroacetyl β-cyclodextrin and twenty one millimole of norfloxacin are dissolved in tetrahydrofuran in presence of N-ethyldiisoprophylamine. The reaction mixture was refluxed for 4-5 hrs, after completion of the reaction mixture cooled to room temperature and then poured in ice water. The final product was filtered, dried and used for different charecterization including biological studies. The IR spectrum of compound in (KBr): acetyl ketone appeard at 1726 (γC=O), 1629 (γC=O cyclic ketone), 3441(γ-OH of acid group), 1502(γC=C aromatic ring) 3060 (γC-H aromatic), 2852- 2922(γC-H aliphatic). Mass spectrum of synthesized compound, molecular ion peak is m/z 8262.0102. 1H NMR (DMSO, TMS, δ, 400 MHz 27°C, 400 MHz), 1.709 (t, 3H, CH3 protons of ethyl groups) J= 4.0 Hz), 1.757 (d, 2H, glugopyranose ring C6 carbon protons) J= 6.4Hz) 2.494 (t 2H piprazine ring CH2 protons near to cd) J = 2.0Hz) 3.398 (s, 2H, acetyl group CH2 protons), 3.648 (t, 2H, piperazine ring CH2 protons) J=2.0Hz) 3.985 (d, 2H, glucopyranose ring C1 Carbon protons), J=5.2Hz), 4.089 (m, 1H, glucopyranose ring C5 carbon proton) J=5.2), 4.122 (m, 1H, glucopyranose ring C3 carbon proton) J = 6.8Hz) 4.555 (m, 2H, glucopyranose ring C4 carbons protons) J=7.6Hz), 4.574 (m, 2H, ethyl CH2 protons) J=6.8Hz) 4.609 (m, 1H, glucopyranose ring C2 carbonProtons), 7.170 (d, 1H, norfloxacin C8 carbon protons) J=7.2Hz), 7.934 (d, 1H, norfloxacin C5 carbon protons)13.6Hz) 8.946 (s, 1H, norfloxacin C2 carbon proton), 15.381 (s, 1H, norfloxacin acid proton). 13C NMR (DMSO, TMS, 27°C, 400MHz, δ, ppm) 25.61 (glucopyranose ring C4 carbon), 51.53 (ethyl CH2 carbon) 14.30 (ethyl CH3 carbon), 58.11 (piperazine ring CH2 carbon near to norfloxacin), 59.55(acetyl group CH2 carbon attached with glucopyranose ring). 63.20 (glucopyranose residue C6 carbon), 166.12 (acid group ketone), 169.94 (acetyl group ketone), 176.14 (norfloxacin ring ketone) 137.17 (norfloxacin C9 carbon), 148.45 (norfloxacin C2 carbon), 44.97 – 63.20 (glucopyranose ring corbon), 105.87 – 148.45 (Fluorine attached norfloxacin ring).
HSQC (DMSO, TMS, 27°C, 400 MHz) spectral correlations like δ 42.5 correlated with δ 4.11; δ 2.53 correlated with δ 40.6; δ 207.1 (>C=O) and 170.1 (ipso carbon); 8.28 ppm corresponding to 1.86 ppm; 52.57 ppm correlated with 2.86 ppm; 35.34 ppm correlated with 3.56 ppm; 49.67 ppm correlated with 3.47 ppm and the peak at 60.93 ppm correlated with 4.207 ppm, confirms the title compound formation.
Table 1: Physical properties of compound 3.
|
Physical properties |
Values |
|
λ-Max value |
277 |
|
Colours |
Light yello |
|
Natures |
Amorphous nature |
|
Micro analysis calc/ found |
C = 58.20, H = 5.23, F = 4.60, N = 10.18, O =21.78. |
|
Solubility |
DMSO |
|
Molecular Weight |
8751.46 g/ml |
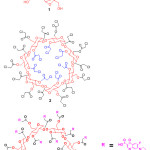 |
Figure 2: Chemical structure of β-cyclodextrine(1), chloroacetyl β-cyclodextrine(2) and β-cyclodextrin core dendrimer(3). |
Synthesis of Compound 5 (Chloroacetyl α-cyclodextrin) and its Spectral Studies
About 0.0011 mol of α-cyclodextrin dissolved in tetrahydrofuran, to the above solution 2.24 mmol of chloroacetyl chloride and N- Ethyldiisopropylamine were added, the reaction mixture was refluxed for 5-6 hrs at 70°C and also the reaction status was monitor by thin layer chromatography. After completion of the reaction, the mixture was poured in ice water and the product (chloroacetyl α-cyclodextrin) separated by low pressure distillation method. Light yellow color semi solid was obtained, before to continue further stages the compound was characterized by IR spectral studies. Presence of carbonyl group stretching frequency at 1739.9cm-1 confirmed the chloroacetyl group. Absence of –OH stretching frequencies conformed that all the hydroxyl group involved in ester formation, this ester bond formation is very useful to drug released study through hydrolysis reaction in aqueous solution with acidic condition.
Synthesis of Compound 6 (Norfloxacin Based α-cyclodextrin Core Drug Delivery System) and its Spectral Studies
A mixture of 4.3 mmol chloroacetyl α-cyclodextrin, 2.25 mmol norfloxacine, 25ml dimethyl sulfoxide and catalytic amount of N-Ethyldiisopropylamine was refluxed at 190°C for 5-6 hrs, after completion of the reaction the mixture was poured into ice water, filtered and dried. The compounds are characterized using IR, UV, 1H and 13C NMR. Glucopyranose ring proton appeared from 2.50 to 3.80 ppm, C1 carbon attached protons chemical shift value is 3.8 ppm. C2 carbon attached protons appeared at 4.1 ppm. C3 carbon attached protons appeared at 3.33 ppm, C6 carbon attached protons appeared at 2.78 ppm, C5 carbon attached protons appeared at 2.5 ppm C4 carbon attached protons appeared at 2.54 ppm. C2 carbon attached proton downfield chemical shift value than other glugopyranose ring protons due to less shielding effect. Acetyl group methylene protons appeared at 2.50 ppm, norfloxacin ethyl group CH3 protons appeared at 1.18 ppm and CH2 protons appeared at 1.31 ppm. norfloxacin aromatic protons appeared from 7.54 to 8.64 ppm, acid group OH proton appeared at 15.20 ppm.
The glucopyranose ring carbon appeared from 40.5 to 106.1ppm, C1 carbon appeared at 106.1 ppm, C2 carbon appeared at 60.3 ppm, C5 carbon appeared at 58.6, C3 carbon appeared at 52.0 ppm, C6 carbons appeared at 50.8. The acetyl CH2 carbon appeared at 49.8ppm, piperazine ring carbons appeared at 45.6 and 50.8ppm, norfloxacin ethyl CH3 carbon appeared at 14.8 ppm. Norfloxacin aromatic carbon appeared from 111.70 to 154.7 ppm. norfloxacin ring unsaturated carbonyl carbon appeared at 176.80ppm, ester carbonyl carbon appeared at 170.36, aced group carbonyl carbon appeared at 166.42 ppm, drug delivery system formation conformed by three carbonyl chemical shift appearance. Piperazine ring two chemically environment carbons appeared at 52.0 and 58.6 ppm, norfloxacin near carbons have less chemical shift values than other two carbons due to the less shielded environment of the carbon. Acid attached and carbonyl presented ring carbons appeared from 107.1 to 148.92, C2 carbon have higher chemical shift value than other carbon of the ring due to less shielded, C3 carbon have less chemical shift value due to acid and carbonyl and ring pi electron strongly shielded, Fluorine attached ring carbons from 106.1 to 152.2 ppm, C8 carbon have up field due to pi electron shielding, fluorine attached carbon have downfield due to fluorine reduced the electron density so carbon is less shielded.
The norfloxacin OH stretching frequencies appeared at 3414.2cm-1, carbonyl group stretching frequencies appeared in the region of 1720 cm-1 , Aromatic stretching frequencies 3019 cm-1 is conformed that presence of norfloxacin, symmetric and asymmetric aliphatic stretching frequencies appeared at 2848 cm-1 and 2954 cm-1. From the UV studies λmax value 277 nm obtained is equal to norfloxacin value and the increasing pH range experiences hypochromic shift and the decreasing pH leads to hyperchromic shift.
 |
Scheme 2: Synthesis of compound 5 (chloroacetyl α- cyclodextrin) and 6 (Norfloxacin based α- cyclodextrin core drug delivery system). |
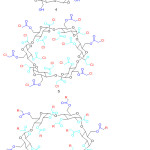 |
Figure 3: Chemical structure of α-cyclodextrine (4), chloroacetyl α-cyclodextrine (5) and β-cyclodextrin core dendrimer (6). |
MALDI TOF Characterization of Compound- 3
MALDI-TOF MS spectrum was used for the determination of molecular mass and the nature of end groups.15,16 MALDI-TOF MS show the number of fragment peaks to synthesized drug delivery system. The most prominent peaks appeared is characterized by fragmentation of different unit from the constructed dendrimer. In total six peaks are appeared at 8262.0102 Da, 7129.1543 Da, 6093.1653Da, 4290.6234Da, 3690.7294Da and 2573.5643 Da in that manner. The molecular ion peak is appeared at 8262.0102 Da (equal to mother ion molecular mass) confirms the formation of titled dendrimer. The expected molecular ion peak value is 8751.10 Da. the polymer unit NOR-CAC mass is 362.33, which unit assign the different fragmentation the twenty one unit are connected with β-cyclodextrin through covalent bond which unit are responsible for different fragment peaks.
The molecular ion peak at 8262.01 Da matching to oligomers doped with H+ of type (C395, H418, F19, N57, O112). The mass peak value at 7129.15 Da corresponding to oligomers doped with H+ of type (C347, H368, F16, N48, O104). The fragment peak appeared at 6093.16Da, matching to oligomers doped with H+ ion of (C283, H304, F12, N36, O92) the above values are confirmed that the norfloxacin fragment generated from drug complex. The peak appeared at 4290.62 (expected value for the fragment is 4323.03) is corresponds to oligomers doped with H+ fragment mass (C203, H224, F7, N21, O77). The peak ranging from 3690.72 Da and 2573.56 Da are corresponds to molecular mass of (C169, H192, F5, N15, O71) and (C118, H144, F2, N6, O62) H+ respectively.
Mechanism of Solubility
Solubility enhancement is important factor to desire therapeutic effectiveness and concentration of drug is systemic movement for pharmacological response.17 the solubility of the norfloxacin highly depend the nature of the solvent. It is almost insoluble in water, alcohol like solvents and shows highest solubility (40 mg/mL) at pH 4–5. Earlier reports in the literature also expose that the solubilisation of norfloxacin drug or HCl bounded norfloxacin in quite a few solvents such as methanol, 2-propanol, etc, is difficult at 27°C and also at elevated temperature.18 Norfloxacin was usually used as an oral treatment for several years. A few previous report are available where norfloxacin is incorporated in various polymer matrices and delivery studies have been carried out.19,20 No studies so far have addressed the possibility for the topical application of newly synthesized norfloxacin based system with biocompatible, biodegradable and antibacterial activity.
Synthesized compound absorption strongly depend the solubility, potency and permeability interaction are essential to develop biological potential of novel compounds. Lipinski et al previously reported that from the commercially available drugs, 87% of the drug solubility is greater than 65 μg/mL.21 They consider that solubility is not likely to limit bioavailability for an orally administered drug with a dose of 1 mg/kg, 65 µg/ml solubility are require to average drug permeability, but bioavailability is limited to solubility is less than 20 μg/mL.22 According to FDA, the aqueous solubility of drug is consider low, their aqueous solubility in 250 mL of pH 1–7.5 aqueous solution is less than the amount contain in a tablet or capsule with the over dosage. Also, according to Biopharmaceutical Classification System (BCS) guidelines, this defines four classes of compounds based on both solubility and choosing permeability.
The discharge rate of norfloxacin from constructed dendrimer structure was determined by using absorption spectrometry. Effect of pH and concentration bring significant change in properties, and indeed, drug solubility and the solubility improvement of a drug increases the odds of successful formulations later in development.23-32 Therefore screening and determining aqueous solubility is one of the most essential steps in drug detection and progress.
A class drugs (solubility 100 μg/mL, high permeability) would greatly improve the bioavailability of the compound.33 The assays and their focal point differ with various phase. It was illustrated with fluoroquinolones drug piperazine ring secondary amine group involved chemical modification. The synthesized polymeric structure of norfloxacin system maintain comparable properties of the parent fluoroquinone, without necessitate of intracellular cleavage and discharge of antibiotic.
The polymeric structures of norfloxacin dendrimer solubility differ with different pH solvent and it is very less soluble in neutral pH water and alcohol. We can use various buffer solutions to find out solubility purpose. If we are decreasing the range of pH from 7 to 1 drug solubility is found to increasing and this is corresponds to the hydrochloride form of norfloxacin. Buffer solutions were prepared from 0.1m HCl with 0.1m sodium bicarbonate solution and the pH value are adjusted with hydrochloric acid (HCl).
Drug Release Studies of Compound 3 and 6
Drug release from the designed system is determined by UV spectrometry with different pH solution and found to give different intensity of the drug concentration. Different pH solution prepared such as pH1, pH2 pH3, pH4, pH6 and pH7 by the adjustment of stock solutions, pH1 buffer solution give higher absorption intensity due to increasing drug release with respect to the increasing acidity.
The microbiological evaluation shows that the discharge of norfloxacin possesses antimicrobial activity. The drug-delivery behaviour of the system was studied to expose the potential use as a drug liberation system. The outcome shows that the norfloxacin release rates of the β-CD + NOR complex could be controlled by the drug loading substance and the liberate medium. Representative examples for amphiphilic drug delivery system as nanocarriers based cyclodextrin are cyclodextrin conjugate, cyclodextrin inclusion complex and pseudopolyrotaxane. Hydrolysis studies were carried out to assess the norfloxacin release from macrocycle β-cyclodextrin under diverse conditions. The in vitro drug release study was investigated by incubation in buffer solution of pH 1, 2,3, 4, 6 and 7, an indicator of this is in the lower pH of higher efficiency compared to neutral pH value (7). The release rate is highly dependent of the ester segment in the drug delivery system against the pH of the medium. Kinetics and mechanistic studies of the biodegradation of polyester is still under investigation and we intend to present and to discuss those findings in a future report. Hydrolysis studies were carried out to evaluate the norfloxacin liberate from the macromolecule in different pH conditions. The collective % of linked drug discharge from the system is revealed in Figure 2. The delivery system undergoes faster norfloxacin release at lower pH with respect to time. At pH-7, only 20% of drug was released in 14 h and about 50–60% drug was released in 14 h at pH-4 and 6. However, the drug released at PH-1 in 14 hrs is about 98%. The hydrolysis of drug at lower pH is rapid and drug release also very fast. The hydrolytic degradation of the drug candidate may also taking place by the cleavage of amide linkage. The above results showed that the drug release is very high at the gastric fluid pH (1.5) in controlled manner. The amount of released norfloxacin from the system was determined by uv spectra at 280 nm, found to result a standard curve by plotting concentration versus absorption (Fig 3).
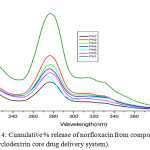 |
Figure 4: Cumulative % release of norfloxacin from compound 3 (β-cyclodextrin core drug delivery system). |
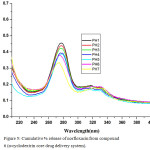 |
Figure 5: Cumulative % release of norfloxacin from compound 6 (α-cyclodextrin core drug delivery system). |
As expected, the sustained drug delivery of synthesized polymer structure directly related to solubility and in the pH1 buffer solution gives higher absorption intensity due to increasing drug solubility. Within 14 hrs the quantity of drug fully functionalized. The uv spectrum conformed that β-cyclodextrin drug delivery system have more difference between pH1 and pH 7 than α-cyclodextrin. β-cyclodextrin drug delivery system is increasing drug delivery on decreasing pH order and the β-cyclodextrin is more convenient to be as good sustain drug delivery system than α-cyclodextrin.
In Vitro Antibacterial Studies
The anti microbiological studies are measure of relative potency and activity of compounds against microorganism under suitable condition. Two different methods are adopted to screen the above assay via disc diffusion and two-fold serial dilution.
Preparation of Media
Growth of the microorganisms depends on favourable environment. Nutrient broth was used to significant cultivation of given microorganism. Agar liquid was prepared from by adding 24% w/v agar in the nutrient broth for making agar slants. Media is a nutritional requirement and then supplies the essential nutrients in the proper form and proportion in a culture media. Bacteria were subculture from suitable nutrient slants. The inoculams was prepared by transferring loopful of the related test organism from the stock bacteria culture into the sterile broth and incubated at 37ºC for bacteria species such as staphylococcus aureus (mtcc 737), Bacillus subtilis (mtcc 2063), Escherichia coli (mtcc-443), pseudomonas aeruginosa (mtcc 741) and Proteus mirabilis (mtcc425). Each petri dish plats contain 20 mL of sterile nutrient agar media and added 2mL of 24 h broth culture if bacteria were, then added to the relevant plates and mixed carefully by rotatory motion of the plates. The synthesized dendrimers were dissolved in DMSO media in the concentration of 10 mg/mL and this was maintained as stock solution. The various concentrations (100, 200 and 500 ppm) has been prepared from the stock solution. A sterile paper disk of 5 mm diameter was saturated with agar plates. The Petri dish plate were incubated with 37ºC and the zone of inhibition was calculated excluding the disc diameter of the paper discs (5 mm) control discs were performed with sterile water.
Anti Bacterial Activity
The norfloxacin surface moiety biological ability was investigated through their in vitro antibacterial ability against various Gram-positive and Gram negative, the Gram-positive bacterial strains like Staphylococcus aureus (mtcc 737), Vibrio cholerae (mtcc-3906) and Bacillus subtilis (mtcc 2063) and the Gram negative bacterial species like Escherichia coli (mtcc-443, Klebsiella pneumonia, Salmonella typhi at a concentration of 100 µg/mL by standard cup plate method and the results, in fact, were compared with a standard drug (Ciprofloxacin).
Table 2: In vitro antibacterial activity of compound 3 (Zone of inhibition in mm).
|
Name of the organism |
ppm |
Staphylococcus aureus |
Vibreo cholera |
Bacillus subtilis |
Escherichia coli |
Klebsiella pneumonia |
Salmonella typhi |
|
Compound 3 |
100 |
30.4 |
30.6 |
31.6 |
32.4 |
30.1 |
31.4 |
|
Ciprofloxacin |
500 |
10.4 |
10.5 |
10.8 |
10.2 |
10.6 |
10.8 |
About 20 ml of liquid agar media freshly prepared and poured into each petridish plate and this plate keep in incubater at 37°C for 1hrs, after 1hrs dried and the microorganism was added to each petridish plate. An L-shaped spreader was used to spread the standardized culture of microorganism on each petridish. Cups size approximately 6 mm diameter cupe were prepared in petridish using sterile cork borer and then labelled. DMSO solvent negative control is also to see the effect of solvent on the growth of the microbes. Dimethylsulfoxide solvent is used to prepare the test solution and standard ciprofloxacin solution (100 µg/mL). The prepared test solution is added to each cup in petridish plate and kept aside in an asidein an aseptic area for 1h to allow diffusion of the drug sample followed by incubation at 37°C for 24 h. The zone of inhibition diameter (in mm) was measured and the results are given the Table 2. Broth dilution method was investigated to evaluate the MIC of selected compounds that inhibited growth of at least one microorganism as per the standard procedure.34
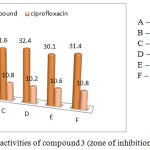 |
Figure 6: Antibacterial activities of compound 3 (zone of inhibition methods). |
Victorious foreword of biologically applicable piperazine ring in some of our previous cases act as important core moiety to produced the higher generation dendrimer through the bond formation between norfloxacin and macromolecule.35 Herewith, we focus on norfloxacin piperazin ring bonded novel drug delivery system synthesis, β-cyclodextrin drug delivery system with twenty one norfloxacin molecule as a outside moiety and also screened for their in vitro antibacterial activity against six strains (three Gram positive and three Gram negative). The zone of inhibitions of the synthesized system 3 is higher than that of ciprofloxacine against different bacteria specieses. Similarly the Escherichia coli zones of inhibition values are very high compared with other bacterias. The zone of inhibition values are given in Figure 2. The remaining bacterial organism’s zone of inhibition values are shown in the Table 2. The final synthesized system 3 showed significant activity against Staphylococcus aureus (mtcc 737), Vibrio cholera (mtcc-3906), Bacillus subtilis (mtcc 2063) (Gram positive) and Escherichia coli (mtcc-443), Klebsiella pneumonia, salmonella typhi (Gram negative). The compound 3 was found the excellent antibacterial activity against total tested organisms. Fig 5 show that the minimum inhibition properties are at low concentration of 0.830 µg/mL-1 against staphylococcus aureus, at 0.950 µg/mL–1 vibreo cholera, at 1.050 µg/mL–1 against Bacillus subtilis, at 0.925 µg/mL-1 against Escherichia coli, at 0.975 µg/mL–1 against Klebsiella pneumonia and at 1.090 µg/mL-1 against Salmonella typhi due to this dendrimer’s twenty one molecules of norfloxacin drug. The MIC values are given in Table 3. It is obvious that the inhibitory effects of the compound 3 more potent than the standard.
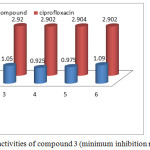 |
Figure 7: Anti bacterial activities of compound 3 (minimum inhibition methods). |
Table 3: In vitro antibacterial activity of compound 3 (minimum inhibition concentration (µg/mL-1)).
|
Name of the organism |
Staphylococcus aureus |
Vibreo cholerae |
Bacillus subtilis |
Escherichia coli |
Klebsiella pneumonia |
Salmonella typhi |
|
Compound 3 |
0.830 |
0.950 |
1.050 |
0.925 |
0.975 |
1.090 |
|
Ciprofloxacin(std) |
2.903 |
2.901 |
2.920 |
2.902 |
2.904 |
2.902 |
MTT Based Cell Viability Assay for Compound 3 (Norfloxacin Based β-cyclodextrin Core Drug Delivery System)
The MTT assay is a colorimetric assay for measuring cell viability through increased metabolism of tetrazolium salt (Moshmann, 1983). Cultured Escherichia coli, staphylococcus aureus were taken into 96 well plates. Then the cells were treated with different concentration of norfloxacin dendrimer (5, 15, 25, 50, 75 and 100µl)and ciprofloxacin (100 µl) were incubated at 37°C for 12, 24, 48 and 72 hrs The MTT (0.5 mg/ml) was added to the cells and then further incubated for another 3 h. The cells were centrifuged for 10 min and the supernatant was removed, 200 μl of dimethyl sulfoxide (DMSO) were added into each tubes. Absorbance was measured in a microplate reader at 560 nm.
Discussion
Antibacterial activity of norfloxacin measured by MTT assay in E.Coli cells (Table 4 and Figure 6) show the cytotoxic effect of various concentrations (5, 15, 25, 50, 75 and 100µl) of norfloxacin drug delivery system in E. coli cells. Among the four different time duration, 72 hrs of norfloxacin dendrimer showed a maximum antibacterial activity when compared to others in E.coli cells. The IC50 value was found to be 75 µL concentrations.
Table 4: Cytotoxicity effect of compound 3 in E. coli cells by MTT assay.
|
Incubation time (hrs) |
Control (%) |
Cip (%) |
Norfloxacin dendrimer (%) |
|||||
|
5 µl |
15 µl |
25 µl |
50 µl |
75 µl |
100 µl |
|||
|
12 |
100 |
90.3245 |
99.975521 |
99.75134 |
98.51487 |
97.86580 |
97.25099 |
94.85149 |
|
24 |
100 |
89.2671 |
99.150623 |
99.11515 |
98.70908 |
89.57513 |
84.13306 |
75.2651 |
|
48 |
100 |
91.3462 |
98.675351 |
95.72077 |
93.71297 |
86.87517 |
82.57885 |
73.56674 |
|
72 |
100 |
90.2345 |
94.521720 |
86.13485 |
82.95809 |
75.41404 |
53.64531 |
40.82042 |
Values are Expressed as the Mean ± SD of Three Experiments in Each Group
The norfloxacin dendrimer exhibited significant antibacterial property in staphylococcus aureus measured by MTT assay. Effect of Norfloxacin dendrimer cytotoxicity in staphylococcus aureus showed a different concentration manner (Table 5 and Figure 7). It has been noticed that the 72 and 48 hrs showed a maximum cytotoxicity against staphylococcus aureus. The IC50 value of norfloxacin was found to be 50 and 100 µL concentrations.
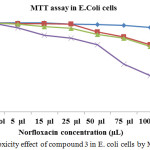 |
Figure 8: Cytotoxicity effect of compound 3 in E. coli cells by MTT assay. |
Data expressed as mean ± SD of three experiments in each group.
Table 5. cytotoxicity effect of compound 3 in Staphylococcus aureuscells by MTT assay.
| Incubation time (hrs) | Contro l(%) | Cip (%) |
Norfloxacin dendrimer (%) |
|||||
|
5µl |
15µl |
25µl |
50µl |
75µl |
100µl |
|||
|
12 |
100 |
90.0164 |
99.97552 |
97.40176 |
89.70395 |
82.90682 |
78.37605 |
73.31369 |
|
24 |
100 |
91.2067 |
94.69339 |
91.352 |
86.66707 |
74.39486 |
69.24674 |
62.67624 |
|
48 |
100 |
91.3672 |
91.56205 |
89.30227 |
83.35587 |
73.78682 |
64.91791 |
41.41115 |
|
72 |
100 |
90.2316 |
91.86951 |
85.24783 |
66.0488 |
39.99823 |
31.63199 |
23.73725 |
Values are expressed as the mean ± SD of three experiments in each group.
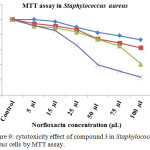 |
Figure 9: cytotoxicity effect of compound 3 in Staphylococcus aureus cells by MTT assay. |
Values are expressed as the mean ± SD of three experiments in each group.
The results of cell viability determination were performed by MTT assay technique showed that the synthesized test compound has remarkable activities against Escherichia coli and staphylococcus aureus. Therefore, they could be introduced as newly synthesized antibacterial agents against commonly occurring microorganism. Moosavy et al., 2008, Fazeli et al., 2007, Misaghi and Akhondzadeh.36,37 were previously reported that the antibacterial activities of some compound. Antibacterial agent tested with various microorganisms such as Salmonella. typhimurium, Staphylococcus aurous and Bacillus cereus in a food model system and in brain heart infusion (BHI) broth.
The current study was illustrating the long time biological efficiency of newly synthesized dendrimer by MTT assay technique. Viable cell percentage changes with respect to different time duration against various concentrations. This result conforms that the sustain delivery of norfloxacin from system. E coli cell viability calculated after incubation with dendrimer for 12, 24, 48 and 72 hrs and the data are given table 4. After 12 hrs incubation the cell viability is 94.25 for 100µl only 5.75 percentage bacteria killed by test compound. After 24 hours incubation the cell viability of E coli 75.26 only 24.74 killed. For 48 hours 100µl show 73.57 cell viable 26.43 percentage only killed. For 72 hours 100µl show 40.82 percentage cell viable 59.82 percentage are killed. The slow decrease in the cell viability conformed sustain delivery of synthesized system. Drug delivery time and concentration is as show in the Figur 6.
Norfloxacin system performed with Staphylococcus aureus to prove the control drug delivery. It is incubated on 12, 24, 48 and 72 hrs with different concentration 5, 15, 25, 50, 75 and 100µl, viable cell percentage differ with respect treated drug concentration. For 12 hrs 100µl give 73.3 percentage viable cells, 26.7 percent only killed by norfloxacin dendrimer. After 24 hrs incubate at 100 µl show 62.7% viable cells, 37.3 % only killed. After 48 hrs at 100 µl show 41.4 percent viable cell. 58.6 % are killed by drug releases. For 72 hrs at 100 µl show 23.7 percent viable cells, 76.3 percent are killed, this result explicit sustain drug delivery of norfloxacin delivery system. Viable cell reduced with increasing incubation time and increasing concentration and the result are given in Table 5. The death percentage of cells for each hour with concentration is given in the Figure 7. Results are compared with standard drug.
MTT Based Cell Viability Assay for Compound 6 (Norfloxacin based α-cyclodextrin Core Drug Delivery System)
Various concentration of (5, 15, 25, 50, 75 and 100 µl) compound 6 (α-cyclodextrin core drug) treated with Escherichia coli species, Cell viability significantly reduced by concentration 50, 75 and 100 µl. IC50 value found to be concentration 25 µl at 24hrs and given Fig 7. Compound 6 reduces the cell viability significantly from 24hrs after that the cell viability increasing to 48 and 72 hrs. viability of Escherichia coli upto 31.6 and 28.6 % at 24 hrs incubation with 75 and 100 µl concentration, the same concentration after 48 and 72 hrs viability increasing to 64.8 and 60.2 %, as mentioned in the Table 7. These results reveal that the α-cyclodextrin core drug delivery system hasn’t long term activity due to sudden releases of drug unit.
Table 6: Cytotoxicity effect of compound 6 in E. coli cells by MTT assay.
|
T( hrs) |
Ctrl |
Std |
Concentration (µl) |
|||||||||
|
5 |
15 |
25 |
50 |
75 |
100 |
|||||||
|
12 hrs |
100 |
61.73395 |
64.88412 |
59.12361 |
55.59152 |
52.17890 |
48.98358 |
45.03259 |
||||
|
24 hrs |
100 |
56.91428 |
63.57932 |
55.216 |
48.07273 |
42.91566 |
31.63199 |
28.60258 |
||||
|
48 hrs |
100 |
87.72871 |
86.02865 |
80.31883 |
77.43022 |
70.06951 |
64.80476 |
60.29364 |
||||
|
72 hrs |
100 |
89.38567 |
92.14360 |
89.96143 |
88.72496 |
86.11791 |
83.54512 |
80.16662 |
||||
Values are expressed as the mean ± SD of three experiments in each group.
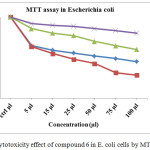 |
Figure 10: Cytotoxicity effect of compound 6 in E. coli cells by MTT assay. |
Values are expressed as the mean ± SD of three experiments in each group.
The IC50 value found to be 50 µl at 24hrs on Staphylococcus aureus as shown in the Fig 8. Compound 6 reduce the cell viability significantly from 24 hrs after the the cell viability increasing to 48 and 72 hrs. viability of Staphylococcus aureus upto 44.1 and 47.7 % at 24 hrs incubation with 75 and 100 µl concentration, after 48 and 72 hrs at the same concentration the cell viability increased to 58.62 and 53.28% (Table 8), from this results α-cyclodextrin core system only active up to 24 hrs so α-cyclodextrin not convenient to design as sustain drug delivery systems.
Table 7: Cytotoxicity effect of compound 6 in Staphylococcus aureuscells by MTT assay.
| T(hrs) | Ctrl | Std | Concentration(µl) | |||||
| 5 | 15 | 25 | 50 | 75 | 100 | |||
| 12hrs | 100 | 65.45035 | 61.66549 | 59.89519 | 56.47952 | 53.17963 | 50.81055 | 68.9145 |
| 24hrs | 100 | 61.59068 | 58.01998 | 54.71916 | 51.77216 | 47.75287 | 44.10301 | 47.6965 |
| 48hrs | 100 | 74.12499 | 71.17041 | 67.56151 | 64.45963 | 60.93185 | 58.62305 | 53.28601 |
| 72hrs | 100 | 95.81835 | 92.34396 | 85.90499 | 79.245 | 72.50545 | 69.70001 | 73.04936 |
Values are expressed as the mean ± SD of three experiments in each group.
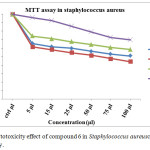 |
Figure 11: Cytotoxicity effect of compound 6 in Staphylococcus aureuscells by MTT assay. |
Values are expressed as the mean ± SD of three experiments in each group.
Conclusion
The synthesised β-cyclodextrin core drug delivery system have long time activity due to their sustain releases of norfloxacin unit is identified by MTT assay method and the results proved that the drug delivery system reduced the cell viability of Escherichia coli and Staphylococcus aureus species. The synthesized β-cyclodextrin core drug delivery system long time activity against both the species is found to slowly reduce the living cells from 12 to 72 hrs. The α-cyclodextrin core released drug activity against both cells is reduced the cell viability from 12 to 24 hours after that cell viabilities are increased from 24 to 72 hrs. From this result the β-cyclodextrin core system act as very good sustain drug delivery system and also it is more convenient to design.
Acknowledgements
The authors express sincere thanks to the head of the Department of Chemistry, Annamalai University, Tamilnadu, India for the facilities provided to carry out this research work.
References
- Uekama, K.; Hirayama, F.; and Irie, T. Chem. Rev., 1998, vol. 98, no. 5, p. 2045; Davis, M.E. and Brewster, M.E., Nature Rev., 2004, vol. 3, p. 1023.
- Uekama, K.; Minami, K.; and Hirayama, F. J. Med.Chem., 1997, vol. 40, no. 7, p. 2755; Minami K, Hirayama F, and Uekama K. J. Pharm. Sci., 1998, vol. 87, no. 6, p. 715; Hirayama F, Kamada M, Yano H, Udo, K, Arima H, and Uekama K. J. Incl. Phenom. Macrocyc. Chem., 2002, vol. 44, p. 159; Yano H, Hirayama F, Kamada M, Arima H, and Uekama K. J. Controlled Release, 2002, vol. 79, nos. 1-3, p. 103.
- Kraus, T. Modified cyclodextrins with pendant cationic and anionic moieties as hosts for highly stable inclusion complexes and molecular recognition. Curr. Org. Chem. 2011, 15, 802–814.
- De Rossi, R. H.; Silva, O. F.; Vico, R. V.; Gonzalez, C. J. Molecular organization and recognition properties of amphiphiliccyclodextrins. Pure Appl. Chem. 2009, 81, 755–765.
- Sallas, F.; Darcy, R. Amphiphiliccyclodextrins-Advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 6, 957–969.
- Korendovych, I. V.; Roesner, R. A.; Rybak-Akimova, E. V. Molecular recognition of neutral and charged guests using metallomacrocyclic hosts. Adv. Inorg. Chem. 2007, 59, 109–173.
- Wang, M. Nitrogen and oxygen bridged calixaromatics: Synthesis, structure, functionalization,and molecular recognition. Acc. Chem. Res. 2012, 45, 182–195.
- Thatiparti, T. R.; Shoffstall, A. J.; von Recum, H. A. Cyclodextrin-based device coatings for affinity-based release of antibiotics. Biomaterials 2010, 31, 2335–2347.
- Liu, Y.; Chen, Y. Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Acc. Chem. Res. 2006, 39, 681–691.
- Easton, C. J.; Lincoln, S. F. Chiral discrimination by modified cyclodextrins. Chem. Soc. Rev. 1996, 25, 163–170.
- Murakami, Y.; Kikuchi, J.; Hisaeda, Y.; Hayashida, O. Artificial enzymes. Chem. Rev. 1996, 96, 721–758.
- Walker, B. D.; Joshi, G.; Davis, A. P. Progress in biomimetic carbohydrate recognition. Cell. Mol. Life Sci. 2009, 66, 3177–3191.
- Goldstein EJC. Am J Med 1987, 82(suppl. 6B), 3.
- Morita, Y.; Kodama, K.; Shiota, S.; Mine, T.; Kataoka, A.; Mizushima, T.; Tsuchiya, T. Antimicrob Agents Chemother 1998, 42, 1778.
- Wachsen, O.; Reichert, K.H.; Kruger, R.P.; Much, H.; Schulz, G. Thermal decomposition of biodegradable polyesters, 3 Studies on the mechanism of thermal degradation of oligo-L-lactide using SEC, LACCC and MALDI-TOF MS. Polymer Degrad Stabil. 1997, 55, 225–231.
- Nielen, M. W. F. MALDI TOF mass spectrometry of synthetic polymers. Mass Spectrometry Rev. 1999, 18, 309–344.
- Shine, A. J. et al,’’ solubilisation of poorly soluble drugs; A Review,’’ pharmainfo. net 2007; 5; 6 [
- Caço, A. I.; Varanda, F.; Pratas de Melo, M. J.; Dias, A. M. A.; Dohrn, R.; Marrucho I. M. Solubility of antibiotics in different solvents. Part II. Non-hydrochloride forms of tetracycline and ciprofloxacin. Ind Eng Chem Res. 2008;47(21):8083–8089.
- Tsou, T. L.; Tang, S. T.; Huang, Y. C.; Wu, J. R.; Young, J. J.; Wang, H. J. Poly(2-hydroxyethyl methacrylate)wound dressing containing ciprofloxacin and its drug release studies. J Materials Sci. 2005;16:95–100
- Unnithan, A. R.; Barakat, N. A.; Pichiah, P. B. et al. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym. 2012;90(4): 1786–1793.
- Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability n drug discovery and development settings, Adv. Drug Deliv. Rev. 23 (1997) 3–25.
- Prentis, R. A.; Lis, Y.; Walker, S. R. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985), Br. J. Clin. Pharmacol. 25 (1988) 387–396.
- Kim, C. K.; Park, J. S. Solubility enhancers for oral drug delivery: canchemical structure manipulation be avoided? Am. J. Drug Deliv. 2 (2004)113–130.
- Chaubal, M. V. Rapid screening of excipients to develop effective formulations, Drug Deliv. Technol. 5 (2005) 40, 42.
- Bittner, B.; Mountfield Richard, J. Intravenous administration of poorly soluble new drug entities in early drug discovery: the potential impact of formulation on pharmacokinetic parameters, Opin. Drug Discov. Dev. 5 (2002) 59–71.
- Gardner, C.; Drug candidate selection: the role of physical chemistry and material science in form and formulation, Bull. Tech. Gattefosse (2005) 9–18.
- Carlson, E. D.; Cong, P.; Chandler Jr, W. H.; Chau, H. K.; Crevier, T.; Desrosiers, P. J.; Doolen, R. D.; Freitag, C.; Hall, L. A.; Kudla, T.; Luo, R.; Masui, C.; Rogers, J.; Song, L.; Tangkilisan, A.; Ung, K. Q.; Wu, L. An integrated high throughput workflow for pre-formulations: polymorph and salt selection studies, Pharma. Chem. 2 (2003) 10–15.
- Gardner, C. R.; Almarsson, O.; Chen, H.; Morissette, S.; Peterson, M.; Zhang, H.; Wang, S.; Lemmo, A.; Gonzalez-Zugasti, J.; Monagle, J.; Marchionna, J.; Ellis, S.; McNulty, C.; Johnson, A.; Levinson, D.; Cima, M. Application of high throughput technologies to drug substance and drug roduct development,nComput. Chem. Eng. 28 (2004) 943–953.
- Gardner, C. R.; Walsh, C. T. Almarsson . Drugs as materials: valuing physical form in drug discovery, Nat. Rev. Drug Discov. 3 (2004) 926– 34.
- Morissette, S. L.; Almarsson, O.; Peterson, M. L.; Remenar, J. F.; Read, M. J.; Lemmo, A. V.; Ellis, S.; Cima, M. J.; Gardner, C. R. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids, Adv. Drug Deliv. Rev. 56 (2004) 275–300.
- Kerns, E. H. High throughput physicochemical profiling for drug discovery, J Pharm. Sci. 90 (2001) 1838–1858.
- Lipper, R. A. How can we optimize selection of drug development candidates from many compounds at the discovery stage? Mod. Drug Discov. 2 (1999)55–60.
- Amidon, G. L.; Lennernaes, H.; Shah, V. P.; Crison, J. R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm. Res. 12 (1995) 413–420.
- Sharma, K. Manual of Microbiology Tools and Techniques, 2nd ed.; Ane’s Book India: New Delhi, 2007.
- Vembu, S.; Pazhamalai, S.; Gopalakrishnan, M. Potential antibacterial activity of triazine dendrimer: Synthesis and controllable drug release properties. Bioorganic Med Chem. 2015;23(15):4561-4566. doi:10.1016/j.bmc.2015.06.009.
- Moosavy, M. H.; Akhondzadeh-Basti, A.; Misaghi, A.; Zahraei-Salehi, T.; Abbasifar R.; Ebrahimzadeh, H.; Mousavi, H. A.; Alipour, M.; Emami Razavi, N.; Gandomi, H.; Noori, N. Effect of Zataria multiflora Boiss essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Res Int. 2008;41:1050–1057.
- Misaghi, A. Akhondzadeh-Basti, A. Effects of Zataria muliflora Boiss essential oil and nisin on Bacillus cereus ATCC 11778. Food Control. 2007;18:1043–1049.

This work is licensed under a Creative Commons Attribution 4.0 International License.









