Density and Coefficients of Jones-Dole Equation for Viscosity of Ternary Solutions Containing Sucrose/NaCl/KCl in Aqueous Glycine/Urea/Thiourea at 25°C
St. Francis de sales college. Seminary Hills, Nagpur. 440006 (MS) India.
Corresponding Author E-mail: georgeroy@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/350255
Article Received on : 22-09-2018
Article Accepted on : 02-04-2019
The density and viscosity of ternary solutions containing Sucrose (non-electrolyte), NaCl and KCl (electrolytes) in aqueous-glycine/urea/thiourea were measured at 25°C. The concentration dependence of viscosity has been studied by Jones-Dole relation and viscosity A and β-coefficients were determined. The comparative account of viscosity β-coefficients for sucrose and salts in different aqueous media has been discussed. The results have been interpreted in terms of different interactions among components of solutions.
KEYWORDS:Jones-Dole Relation; Molecular Interaction; Viscosity β-Coefficient
Download this article as:| Copy the following to cite this article: George R. Density and Coefficients of Jones-Dole Equation for Viscosity of Ternary Solutions Containing Sucrose/NaCl/KCl in Aqueous Glycine/Urea/Thiourea at 25°C. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: George R. Density and Coefficients of Jones-Dole Equation for Viscosity of Ternary Solutions Containing Sucrose/NaCl/KCl in Aqueous Glycine/Urea/Thiourea at 25°C. Orient J Chem 2019;35(2). Available from: https://bit.ly/2DAGLYu |
Introduction
Viscometric studies of transport properties give significant information regarding molecular interactions and structural fittings in aqueous solution. P. C. Dey et al. have studied volumetric and viscosity studies of carbohydrates in solutions,1 A. A. Ansari et al. studied volumetric and viscometric study of glucose in aqueous solution2 and R. Mathpal et al. have studied intermolecular forces of sugars in water.3 Sucrose is a most common disaccharide obtained from sugar beets and sugarcane. Sucrose is biochemically important. It plays important role in living systems in storage and transport of energy and in immune-system. It is a non-electrolyte polar substance having hydrophilic hydroxyl group and associated with hydrogen bonding.
Therefore, in view of its importance and structural features, it becomes essential to understand the structure and molecular interaction in aqueous solutions of sucrose. Solute-solvent interactions in carbohydrate/sucrose solutions have been studied by many workers.4-8 Partial molar volumes of mono and disaccharides have been studied by Banipal et al.9
Studies of molecular interactions in binary aqueous sucrose and ternary sucrose + aqueous glycine/urea/thiourea are highly important for research and industrial purpose. S. D. Deosarkar et al. have studied the molecular interactions in solutions of para-substituted benzoic acids, potassium salt and aqueous duloxetine solutions.10-12 Here we report, a comparative study of viscosity B-coefficients of sucrose, NaCl and KCl in different aqueous media which is lacking to the best of our knowledge.
Experimental
All glassware used during experiments was of Borosil make and calibrated before use. Weighing was done on single pan electronic balance (±0.0001 g). The HPLC grade deionized distilled water obtained from Millipore prefiltration kit (Direct-QTM system series) was used for preparation of solutions. Sucrose solutions were prepared using double distilled water in stock solutions of aqueous-glycine/urea/thiourea (0.001 mol∙dm-3) in calibrated volumetric flasks by dissolving accurately weighed solute. Densities were measured by single capillary pycnometer of different volumes and averages of three readings are reported. Viscosities were determined from Ostwald type viscometer by flow time method. The temperature water bath was used for maintaining constant temperature of experimental solution (±0.1°C).
Results and Discussion
Experimental densities (r), viscosities (h), and Jones-Dole viscosity (hr-1/√c) for NaCl, KCl and sucrose in aqueous-{glycine/urea/thiourea) solutions at 25°C are reported in Table 1 and 2. It is seen that in each case density and viscosity increased with concentration of sucrose in solution which clearly indicates modification of structural arrangements in solution and existence of interactions between components of solution. Densities show satisfactorily linear dependence (for most of the cases R2 > 0.998) on concentration of solute in each case. Viscosities do not show satisfactorily linear dependence (for most of the cases R2 < 0.988) on concentration of solute in each case. Viscosity was calculated from flow times and density of solution using standard relation.13-14 Viscosity data has been analyzed by Jones-Dole, hr-1/√c=A + B × √c equation. Viscosity A and B-coefficients which represent solute-solute and solute-solvent interactions are determined as intercept and slopes of the plots ofhr-1/√c vs. √c respectively.15-16 Viscosity data has also been analyzed by modified Jones-Dole hr=1 + B × c (Figure 1-3).
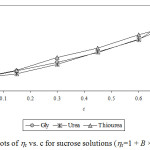 |
Figure 1: Plots of hr vs. c for sucrose solutions (hr=1 + B × c equation). |
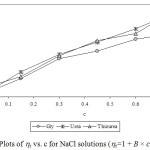 |
Figure 2: Plots of hr vs. c for NaCl solutions (hr=1 + B × c equation). |
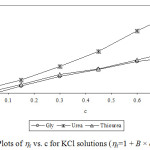 |
Figure 3: Plots of hr vs. c for KCl solutions (hr=1 + B × c equation).Click here to view figure |
Table 1: Density (r), viscosity (h), and Jones-Dole viscosity (hr-1/√c) for Sucrose, NaCl and KCl in different aqueous media at 25°C.
|
c |
r |
h |
hr-1/√c |
|
Sucrose + aqueous glycine |
|||
|
0.05 |
1.0032 |
1.017 |
0.331 |
|
0.15 |
1.0162 |
1.112 |
0.452 |
|
0.30 |
1.0358 |
1.263 |
0.611 |
|
0.45 |
1.0548 |
1.437 |
0.773 |
|
0.60 |
1.0753 |
1.675 |
0.993 |
|
0.75 |
1.0945 |
1.974 |
1.253 |
|
Sucrose + aqueous urea |
|||
|
0.05 |
1.0039 |
0.998 |
0.244 |
|
0.15 |
1.0157 |
1.062 |
0.314 |
|
0.30 |
1.0352 |
1.228 |
0.543 |
|
0.45 |
1.0551 |
1.447 |
0.789 |
|
0.60 |
1.0748 |
1.670 |
0.986 |
|
0.75 |
1.0948 |
2.134 |
1.449 |
|
Sucrose + aqueous thiourea |
|||
|
0.05 |
1.0041 |
1.028 |
0.383 |
|
0.15 |
1.0166 |
1.127 |
0.492 |
|
0.30 |
1.0352 |
1.349 |
0.777 |
|
0.45 |
1.0552 |
1.519 |
0.902 |
|
0.60 |
1.0753 |
1.758 |
1.106 |
|
0.75 |
1.0939 |
1.951 |
1.225 |
Foot Note: c=mol∙dm-3; r=g∙cm-3 and h=mPa∙s.
These coefficients are reported in Table 3. The plots ofhr-1/√c vs. √c showed non-linear behavior for higher concentration of solute.
The values of viscosity B-coefficient are positive for all systems which indicate existence of strong solute-solvent or ion-solvent interactions in solution. The B-coefficients for aqueous solutions of sucrose in different media are larger than for salts and B-coefficients for aqueous solutions of KCl in different aqueous media are larger than for NaCl. This indicates more structural organization of sucrose solutions compared to NaCl and KCl solutions. The B-coefficients for all the solutes viz. sucrose, NaCl and KCl in aqueous urea solutions are larger than in any other aqueous medium due to more structural organization17 and relatively strong solute-solvent or ion-solvent interactions in sucrose/NaCl/KCl + aqueous urea solutions. The A-coefficient is either negative or positive but very small for all the systems due to weak ion-ion or solute-solute interactions. For NaCl in all the aqueous media, coefficient A is positive due to existence of ion-ion or interactions.
Table 2: Density (r), viscosity (h), and Jones-Dole viscosity (hr-1/√c) for Sucrose, NaCl and KCl in different aqueous media at 25oC.
| c | r | h | hr-1/√c | r | h | hr-1/√c |
| NaCl + aqueous glycine | KCl + aqueous glycine | |||||
| 0.05 | 0.9982 | 0.958 | 0.053 | 0.999 | 0.947 | 0.001 |
| 0.15 | 1.0016 | 0.973 | 0.073 | 1.0037 | 0.964 | 0.046 |
| 0.3 | 1.0079 | 1.002 | 0.106 | 1.0109 | 0.986 | 0.075 |
| 0.45 | 1.0137 | 1.012 | 0.103 | 1.0179 | 1.003 | 0.088 |
| 0.6 | 1.0196 | 1.029 | 0.112 | 1.0254 | 1.021 | 0.102 |
| 0.75 | 1.0264 | 1.038 | 0.111 | 1.033 | 1.032 | 0.104 |
| NaCl + aqueous urea | KCl + aqueous urea | |||||
| 0.05 | 0.999 | 0.961 | 0.068 | 0.9997 | 0.962 | 0.071 |
| 0.15 | 1.002 | 0.982 | 0.098 | 1.0043 | 0.978 | 0.086 |
| 0.3 | 1.0072 | 1.008 | 0.118 | 1.0108 | 1.009 | 0.121 |
| 0.45 | 1.0132 | 1.025 | 0.124 | 1.0173 | 1.045 | 0.155 |
| 0.6 | 1.0193 | 1.045 | 0.134 | 1.0249 | 1.094 | 0.201 |
| 0.75 | 1.0271 | 1.067 | 0.146 | 1.033 | 1.137 | 0.232 |
| NaCl + aqueous thiourea | KCl + aqueous thiourea | |||||
| 0.05 | 0.999 | 0.965 | 0.087 | 0.9997 | 0.953 | 0.029 |
| 0.15 | 1.0023 | 0.976 | 0.08 | 1.0034 | 0.966 | 0.054 |
| 0.3 | 1.0082 | 1.005 | 0.112 | 1.0105 | 0.99 | 0.084 |
| 0.45 | 1.015 | 1.027 | 0.127 | 1.0175 | 1.004 | 0.09 |
| 0.6 | 1.0208 | 1.037 | 0.123 | 1.0243 | 1.025 | 0.107 |
| 0.75 | 1.0273 | 1.068 | 0.148 | 1.0316 | 1.046 | 0.121 |
Foot Note: c=mol∙dm-3; r=g∙cm-3 and h=mPa∙s.
Table 3: Viscosity A and B-coefficient values for Sucrose, NaCl and KCl in different aqueous media at 25oC
|
System |
A (dm3/2∙mol-1/2) |
B (dm3∙mol-1) |
B (dm3∙mol-1) |
|
|
hr-1/√c=A + B × √c |
hr=1 + B × c |
|
| Sucrose + Aqueous Gly |
-0.063 |
1.380 |
1.322 |
| Sucrose + Aqueous urea |
-0.305 |
1.774 |
1.409 |
| Sucrose + Aqueous thiourea |
0.026 |
1.362 |
1.406 |
| NaCl + Aqueous Gly |
0.039 |
0.094 |
0.143 |
| NaCl + Aqueous urea |
0.049 |
0.114 |
0.176 |
| NaCl + Aqueous thiourea |
0.056 |
0.099 |
0.175 |
| KCl + Aqueous Gly |
-0.023 |
0.159 |
0.126 |
| KCl + Aqueous urea |
-0.004 |
0.257 |
0.255 |
| KCl + Aqueous thiourea |
0.00001 |
0.140 |
0.162 |
In aqueous glycine solution strong solute-solvent interactions exist between polar parts of water and zwitterionic centers of glycine. Hydrogen bonding involving interactions (solute-solute) is broken upon addition of sucrose in water and new network of hydrogen bonding involving (solute-solvent) interactions is formed and sucrose molecule gradually gets hydrated. In glycine + aqueous sucrose solution, the hydrophilic-ionic group interactions (between –OH group of sucrose and zwitterionic centers of glycine) exists apart from sucrose-water interactions. The sucrose acts as structure maker for aqueous-glycine solution.18 Similarly, strong solute-solvent interactions exist between polar water and hydrophilic parts of urea in aqueous urea/thiourea solutions. In sucrose + urea/thiourea aqueous solution, hydrophilic-hydrophilic interactions between –OH group of sucrose and hydrophilic parts of urea/thiourea exists through hydrogen bonding. Sucrose acts as structure maker for aqueous urea and aqueous thiourea solutions.18
Conclusion
Density and viscosity of NaCl, KCl and sucrose in aqueous-{glycine/urea/thiourea) solutions have been measured at 298.15 K. The viscosity A and B-coefficients of sucrose were determined form the Jones-Dole relation. Weak solute-solute interaction as compared to solute-solvent interactions is observed from the viscosity coefficients. The hydrophilic-hydrophilic, ion-hydrophilic, ion-ion/dipole interactions exist between the solutes and cosolute in solution.
Acknowledgements
Author is thankful to Dr Santosh D Deosarkar of School of Chemical Scienecs S.R.T.M. University Nanded for his guidance .Author is also thankful to The Director Laxmi Narayan Institute of technology Nagpur for providing necessary facilities for the work
References
- Dey, P. C. ; Motin, M. A. ; Biswas, T. K.; Huque, E. M. Monatsh. Chem., 2003, 134, 797.
- Mathpal.,R ; Joshi. B. K. ; Joshi, S.; Kandpal, N. D. Monatsh. Chem., 2006, 137, 375-379.
- Khanuja, P.; Chourey, V. R. ; Ansari, A. A. J. Chem. Pharm. Res., 2012, 4, 3047-3050.
- Jahangirdar. D. V.; Lokhande, B. R.; Mehrotra, S. C. Ind. J. Chem., 1995, 34A, 462-.
- Dash N. Patnaik E. R. Ind. J. Chem., 1995, 34A, 834.
- Geeta. D. C.; Rakkppan, Ind. J. Phys., 2003, 77, 525.
- Khanuja, P. ; Chourey. V. R.; Ansari, A. A. Der Chemica Sinica, 2012, 3, 948-952.
- Fucaloro, A. F.; Pu, Y.; Cha, K.; Williams, A.; Conrad, K. J. Solution Chem., 2007, 36, 61-80.
- Banipal, P.K.; Banipal, T.S.; Lark, B.S., Ahluwalia, J.C., J. Chem. Soc., Faraday Trans. 1997 93, 81-87.
- Deosarkar S. D.; Shaikh, U. B. ; Russian Journal of General Chemistry, 201312 2392–2394
- Deosarkar, S. D.; Pandhare V. V. ;. Kattekar. P. S. Journal of Engineering, 2013, 1-4
- Deosarkar. S. D; Deoraye. S. M.; Kalyankar. T. M. ; Russian Journal of Physical Chemistry A, 2014, 88(7) 1129–1132.
- Wang, J. ; Wang. F.; Zhang. P.; Li. C.; Ren. B., J. Chem. Eng. Data, 2008, 53, 648.
- Li H.; Wang. H.; Zhao. L.; Chen. X.; Russ. J. Phy. Chem. A, 2011, 85, 2426.
- Jones G.; Dole. M.; J. Am. Chem. Soc., 1929, 51, 2950.
- Agrawal P. S.; Aust. J. Basic Appl. Sci., 2010, 4, 6519.
- Shaikh. M.; Mohd S.; Farooquic M.; J. Adv. Sci. Res., 2011; 2(2): 21-26.
- Ştefaniu. A.; Iulian O.; Ciocirlan. O., Rev. Roum. Chim., 2011, 56, 869-874.

This work is licensed under a Creative Commons Attribution 4.0 International License.










