Antioxidant Activity of Multifarious Solvent Extracts of Leaf. (Plumbago Zeylanica)
D. Roselin Jenifer*1 and B. R. Malathy2
and B. R. Malathy2
1Department of Bioinformatics, Faculty of Bio and Chemical Engineering, Sathyabama institute of science and technology, Chennai -119, India.
2Department of Microbiology, Reader, Sathyabama Dental College and Hospital, Chennai – 119, India.
Corresponding Author E-mail: jeni.christ20@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350230
Article Received on : 15-12-2018
Article Accepted on : 14-03-2019
Article Published : 17 Apr 2019
The current investigation is focused to determine antioxidant activities of the leaf extract of Plumbago zeylanica. The leaf extracts were prepared by using multiform solvents with increasing polarity, including as hexane, chloroform, ethyl acetate and Methanol. The antioxidant activities enlisting DPPH assay, hydrogen peroxide assay, reducing power assay and nitric oxide assay method were performed for all extracts. Barring no one, the extracts had shown average to potent antioxidant activity. Uniquely, the ethyl acetate extract and the chloroform extract explicated the utmost antioxidant activity.
KEYWORDS:Antioxidant Activity; DPPH Assay; Hydrogen Peroxide Assay; P. Zeylanica; Reducing Power Assay and Nitric Oxide Assay
Download this article as:| Copy the following to cite this article: Jenifer D. R, Malathy B. R. Antioxidant Activity of Multifarious Solvent Extracts of Leaf. (Plumbago Zeylanica). Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Jenifer D. R, Malathy B. R. Antioxidant Activity of Multifarious Solvent Extracts of Leaf. (Plumbago Zeylanica). Orient J Chem 2019;35(2). Available from: https://bit.ly/2GjWFal |
Introduction
In this study antioxidant properties of the leaf extract has been studied. Antioxidants counteract oxidative stress created by free radicals. A Free radical is any molecule that contains an unpaired electron in an atomic orbital and they are unstable and highly reactive by donating or accepting an electron from other molecules, thereby behave as oxidants or reductants (Cheeseman et al., 1998) They cause damage to biological relevant molecules such as DNA, proteins, carbohydrates, and lipids [Young et al., 2001] Cardiovascular disorder, cancer, osteoporosis and degenerative disease caused by the production of reactive oxygen species and oxidative stress (Halliwell et al., 1994). Unsaturated fatty acid induced by free radicals in the membranes, leads to membrane lipid peroxidation, decrease the membrane fluidity, reduction of enzyme receptor activity and damage to membrane protein, which finally triggers the cell inactivation or death (Li.X.M et al., 2007).
Oxidative processes maintained by antioxidants protect the cell from oxidative stress or it creates reactive metabolites Antioxidants act as a blocker or react against free radical to inhibit the toxic effect, scavenge and destroy the physiological system of free radical. Natural antioxidant explored from medicinal plants would be a replacement of synthetic antioxidants, which were restricted due to side effect such as carcinogenicity. The present study focused on Plumbago zeylanica leave extract using four different solvents based on polarity and its antioxidant activity.
Material and Methods
Solvent Extraction of Plant
The leaf of P.zeylanica collected from homoeopathic store, wash the leaf and Shade dried for 15 days. Once grind the dried leaf, 100g of leaf powder combined with 200ml solvents as per polarity (Hexane, Chloroform, Ethyl acetate and Methanol) and blend the mixture to placed on orbital shaker at room temperature for 72 hours. Afterward collect the filtrate by what mann no-1 filter paper, then extract were concentrated to evaporate the solvent by rotary evaporator at low temperature under medium pressure condition. The dried crude extract was stored in airtight container for further experiment and analysis.
In Vitro Antioxidant Activity Assay
Antioxidant Activity by DPPH Assay
Free radical scavenging activity of various plant extracts is measured by the DPPH assay method. DPPH (1,1-Diphenyl-2-picrylhydrazyl) is a stable free radical, which is purple in color and its observance is measured at 517nm. Antioxidant present in the plant extract reacts with DPPH and it was reduced to produce unstable DPPH-H, which is hydrogen donor and its decolorized to form yellow color. Increase decolorization indicates higher reducing the ability of plant extract (Harborne et al., 1998). The varying concentration of plant extracts (25µl, 50µl, 75µl, and 100µl) was added with DPPH (0.1 mm) in methanol solution and kept at room temperature for 30 min. The absorbance was carried out at 517nm by UV –spectrometer (Ahmedetal., 2013). The percent of DPPH scavenging activity was calculated according to the following equation (Acholaetal., 1998).
% of inhibition= Absorbance (control) – Absorbance (sample) / Absorbance (control) × 100
Reducing Power Assay
The components which has reduction potential in plant extract react with potassium ferric cyanide [Fe3+] to reduce to form potassium ferrocyanide [Fe2+], which then combined with ferric chloride to produce ferric ferrous complex, whose Absorbance was measured at 700nm by UV spectrometer. Increased level of observance indicates increased, reducing components present in plant extracts (Jayanthy et al). Potassium ferric cyanide was added to plant extracts. It was incubated at 50°C water bath for 20 min. Then 10% trichloro acetic acid was added and centrifuged at 3000rpm for 10 min. Supernatant was collected and equal volume of distilled water was added. Finally, ferric chloride was added and absorbance was measured at 700nm in the UV – spectrometer.
% of inhibition = Absorbance (control) – Absorbance (sample) / Absorbance (control) ×100
Nitric Oxide Scavenging Activity
Nitric oxide is an important chemical intermediate, generated by endothelial cell. Nitric oxide is an unstable molecule, under aerobic condition, it combines with oxygen to produce stable compound nitrate and nitrite (Rozina parole., et al), which act as free radical that can affect the physiological process of cell. The antioxidant present in plant extract inhibits the nitrite ion formation. The Quantity of nitrite ion was determined by using Griess reagent. Sodium nitroprusside (10mM) in phosphate buffer saline was combined with various concentrations of P. Zeylanica extract and incubated at 30°C for 2 hours 30 min. Finally, Griess reagent was added and Chromophore produced was identified by UV-spectrometer at 590nm.
% of inhibition = Absorbance (control) – Absorbance (sample) / Absorbance (control) × 100
Hydrogen Peroxide Scavenging Assay
The Hydrogen peroxide molecule is not toxic in nature. When it’s undergone several enzymatic activities, it can convert to toxic substance such as hydroxyl radicals. In this assay, antioxidant components present in plant extract inhibits free radical formation and Absorbance was carried out by UV spectrometer at 230nm. The hydrogen peroxide (20mM) in phosphate saline buffer was added to plant extracts in 1:1 ratio and incubated at room temperature for 30 min. It was vortexes and the absorbance was read by UV-spectrometer at 230nm.
% of inhibition = Absorbance (control) – Absorbance (sample) / Absorbance (control) × 100
Results and Discussion
Antioxidant Assay Method
The antioxidant activity of various solvent extracts of P. Zeylanica was calculated to determine the free radical property by DPPH radical assay, Reducing power assay, Nitric oxide assay and Hydrogen peroxide assay. Here, the percentage of inhibition declared the antioxidant concentration of plant extracts. Hence, IC50 values were determined for all Assay models.
Antioxidant Activity by DPPH Assay
The free radical scavenging property of various solvent extracts was analyzed by DPPH scavenging assay. The change in color of DPPH from purple to yellow indicate positive reaction. Though all four solvent showed the presence of antioxidant compound, the ethyl acetate extract was considered superior because it’s IC50 values were 20.62, 32.38, 39.86, 21.3, and 29.92 µg/ml for Hexane, chloroform, ethyl acetate, methanol and ascorbic acid, respectively (Table 1 and Graph-1).
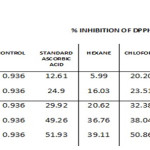 |
Table 1: Dpph Assay. |
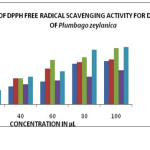 |
Graph 1: Dpph Assay. |
Reducing power assay
When antioxidant donates the electron to free radicals, it gets neutralized. The direct electron donor was measured by reducing power assay, in the reduction of Fe3+ (CN–) 6-Fe2+ (CN–)6 (Yen et al., 1995). The neutralized radical color was Prussian blue and it was measured at 700nm. Ethyl acetate extract showed high antioxidant potential. The IC50 values were 34.8, 66.2, 81.3, 46.5 and 53 µg/ml for Hexane, chloroform, ethyl acetate, methanol extracts and ascorbic acid respectively (Table 2 and Graph-2).
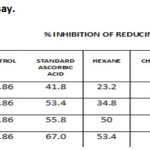 |
Table 2: Reducing Power Assay. |
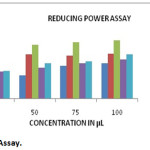 |
Graph 2: Reducing Power Assay. |
Nitric Oxide Scavenging Activity
Nitric oxide scavenger activity is a dominant multitype inhibitor of the physiological process of cell, such as neuronal signaling, smooth muscle relaxation, effect of platelet aggregation and induce the toxicity of the cell. Nitric oxide is a widespread free radical, which commence effector molecule in diverse biological system carrying vasodilation, neuronal messenger, antimicrobial and antitumor activities (Hagerman, et al., 1998).
Nitric oxide scavenging activity result was given in Table 3 & Graph-3 and the IC50 values were 22.1, 39.5, 53.2, 36.8, 57.5 µg/ml for hexane, chloroform, ethyl acetate, methanol and ascorbic acid respectively. Among the four solvent extracts tested ethyl acetate extract showed high nitric oxide scavenging activity.
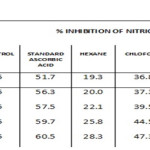 |
Table 3: Nitric Oxide Assay. |
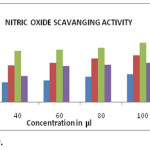 |
Graph 3: Nitric Oxide Assay. |
Hydrogen Peroxide Scavenging Assay
Hydrogen peroxide is an oxidizing agent, but it’s weak in nature and can deactivate several enzymes by oxidation of thiol (-SH) groups. Hydrogen peroxide may cross over the cell membrane and in the cell, hydrogen peroxide may immediately react with Fe2+, and probably Cu2+ to produce hydroxyl radical, which may initiate several toxic effects inside the cell (Kumaran et al., 2007).
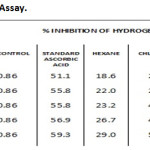
IC50 values were 23.2, 41.8, 53.4, 39.5 & 55.8 µg/ml for hexane, chloroform, ethyl acetate, methanol extract and ascorbic acid respectively. Ethyl acetate extract showed high Hydrogen peroxide scavenging activity (Graph 4 & Table-4).
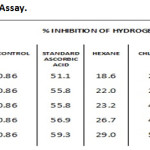 |
Table 4: Hydrogen Peroxide Assay. |
 |
Graph 4: Hydrogen Peroxide Assay. |
Conclusion
Antioxidant properties of four solvent extracts showed, Ethyl acetate to be superior in its scavenging property for free radicals. Hence this study concludes that the ethyl acetate extract can be subjected to further study.
Acknowledgements
I would like to express my deep gratitude to Dr.B.R. Malathy, my research supervisor, for patient guidance and encouragement of this research work.
References
- Cheeseman, K.H.; Slater, T.F.; An introduction to free radical biochemistry. Bare MED Bull. 1993, 49(3),481–93.
- Young, I.S.; Woodside, J.V; Antioxidants in health and disease. J ClinPathol. 2001, 54:176–86.
- Halliwell, B.; Gutleridge, M.; Review article. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical journal. 1984, 219,1-4.
- Li, X.M.; X.L.Li.; Zhou, A.G.; Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruit in vitro. European polymer journal. 2007, 43, 488-497.
- Harborne, J.B.;. Phytochemical methods- A guide to modern techniques of plant analysis, 3rd, Springer (India) Pvt. Ltd, 1998, 5-32.
- Ahmad*, M.; Saeed, F.; Mehjabeen, Noor jahan.; Evaluation of Insecticidal and Antioxidant activity of selected Medicinal plants. Journal of Pharmacognosy & Phytochemistry. 2013, 2(3), 153-158.
- Achola, K.J.; Munenge, R.W.; Bronchodilating and uterine activities of Ageratum conyzoides extract, Pharmaceutical Biology. 2004, 36(2), 93-96.
- Jayanthi, P.; Lalitha, P.; Reducing the power of the solvent extracts of Eichhornia creeps, International Journal of Pharmacy and Pharmaceutical Sciences. 2011,3, 0975-1491.
- Rozina Parul, Sukalayan Kumar Kundu, Pijush Saha, In Vitro Nitric Oxide Scavenging Activity of Methanol Extracts of Three Bangladeshi Medicinal Plants, The pharma innovation-journal. 2012, 12,83-88.
- Yen, G.C.; Chen, H.Y.; Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agriculture and Food Chemistry. 1995, 43, 27–32
- Hagerman, A.E.; Riedl, K.M.; Alexander, J.G.; civic, K.N.; Richard, Nicole, T.; Hartzfeld, P.W.; Rachel, T.L.; High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric. Food Chem. 1998, 46, 1887-1892.
- Kumaran, A.; Karunakaran, R.J.; In vitro antioxidant activities of methanol extract of Phyllanthus species from India. LWT- Food Science and Technology. 2007, 40, 344-352.

This work is licensed under a Creative Commons Attribution 4.0 International License.









