Adsorptive Behavior of Medicinal Product Based Activated Carbon for Removal of Pharmaceutical Active Compounds in Aqueous Phase. Redlich-Peterson Studies
Nsar Sherko Omer, Shameran Jamal Salih* and Hawar Jalal Sadiq Hawezy
and Hawar Jalal Sadiq Hawezy
Department of Chemistry, Faculty of Science and Health, Koya University Park, Danielle Mitterrand Boulevard, Koya KOY45, Kurdistan Region of F.R. Iraq.
Corresponding Author E-mail: shameran.jamal@koyauniversity.org
DOI : http://dx.doi.org/10.13005/ojc/350244
Article Received on : 11-08-2018
Article Accepted on : 15-03-2019
Article Published : 12 Apr 2019
Medicinal product based activated carbon was applied for the sorption of pharmaceutical active compounds in batch and aquatic systems. The MP-AC was characterized utilized X-Ray powder diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM). The sorption process was found to be strongly dependent on the pH of solution which shows maximum sorption efficiency and sorption capacity at lower pH (pH=4) of 89.53% and qe = 29.35 mg.g-1, respectively. Moreover, the pHpzc also determined using drift method to evaluate the surface charge of the sorbent (pHpzc= 6.68). The sorption mechanism suitably described using a Redlich–Peterson isotherm model and confirmed by the value of chi-square test(X2 = 0.01905). The results revealed that the sorption process is spontaneous and more effective at low temperatures.
KEYWORDS:Aquatic System Treatment; Medicinal Product; Redlich-Peterson Model; Sorption
Download this article as:| Copy the following to cite this article: Omer N. S, Salih S. J, Hawezy H. J. S. Adsorptive Behavior of Medicinal Product Based Activated Carbon for Removal of Pharmaceutical Active Compounds in Aqueous Phase. Redlich-Peterson Studies. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Omer N. S, Salih S. J, Hawezy H. J. S. Adsorptive Behavior of Medicinal Product Based Activated Carbon for Removal of Pharmaceutical Active Compounds in Aqueous Phase. Redlich-Peterson Studies. Orient J Chem 2019;35(2). Available from: https://bit.ly/2Z38Tg5 |
Introduction
Countless studies reported the presence of trace amount of pharmaceutical productions in aquatic environment, worldwide, due to discharged into the aquatic bodies that has highly solubility in water.1 Moreover, many of these active compounds have adverse effect on the mankind. Recent studies detected in traces at ng.L-1 to µg.L-1 levels in surface water and industrial wastewater.2,3 Many methods has been applied for pharmaceutical wastewater treatments and found that techniques were helpless due to degrading most these PACs. Thus, residual traces remain in the aquatic system.4 Nevertheless, adsorption technique is a versatile treatment system practiced widely for regulating mobility of pharmaceutical active compound species and their geochemical cycles in the environment.5 The adsorption utilize activated carbon is ideal for removing small molecular compounds due to the availability of high surface area, and combination of well-developed pore structure and surface functional group properties.5,6
Based on literature, ibuprofen, a non-steroidal and anti-inflammatory, is one of the most common drugs found in water6 the latter been detected in the effluents from several sewage-treatment plants at concentrations up to 24.6 µg/L7 possibly presenting a potential hazard for human health.11 Furthermore, virtually all drugs especially in large doses or when taken over long periods, can initiate a toxic condition.8 The major principles applied in the emergency treatment of accidental poisoning drug are dilution, emesis and adsorption. Many types of adsorbents such as kaolin,21 attapulgite and betonies in the prevention of further adsorption of drugs are recognizing in clinical practice and environmental treatment. Adsorption of ibuprofen was studied using activated carbon.23
Meanwhile, Activated carbon, in a powdered form, should be in every medicine cabinet and first aid kit. It is also known as activated charcoal.12 It is used around the world as a universal antidote for hundreds of poisons, including arsenic, mercury, pesticides, strychnine, warfarin, hemlock, E. Coil endotoxin, and gasoline. Over 5,000 chemicals, drugs, plant and microbial toxins, allergens, venoms, and wastes are effectively neutralized by activated charcoal, when it is given in sufficient quantities. Activated charcoal is also an effective detox for practically any drug overdose if administered in time.15
Here we attempt to find a new sorbent to act as physical antidotes in treatment of poisoning if the drug is taken in quantities higher than the recommended dosages, hence to understand that the effect of the some parameters on the sorption process was studied.
Finally, various isotherms (Langmuir, Freundlich, and Redlich-Peterson) and parameters have been examined such as effect of initial IBU solution, pH, contact time, ionic strength and temperature was investigated of loaded IBU onto medicinal products based activated carbon (MP-AC).
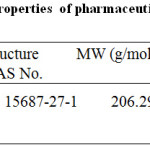 |
Table 1: Physico-chemical properties of pharmaceutical active compound-ibuprofen (PAC-IBU).18,25Click here to view table |
Material and Methods
All reagents used were of analytical purity, Ibuprofen (HIKMA), hydrochloric acid 37% (Analar), potassium chloride (Aldrich), sodium hydroxide(Merk), sodium hydrogen carbonate (Sigma -Aldrich), potassium hydrogen phthalate (Merck) and potassium hydrogen phosphate (Merck) were used to prepare the buffer solutions with different pH values, In addition . All working solutions were prepared by diluting the stock solutions with deionized water.
Preparation of Stock Solution
The stocks were prepared by dissolving 0.1g of ibuprofen in 100 ml using sensitive weight balance (HTCE 3000g). The solution stirred thoroughly with a magnetic stirrer overnight, until a well-mixed solution achieved with all IBP dissolved19. Solutions of known initial concentrations were then created from the stock solution for each experimental treatment. This was done by pipetting the stock solution into vials with ultrapure water. The pHs of the samples were adjusted by the drop-wise addition of HCl or NaOH and the use of an pH meter (pH/Ion 510).
Batch Adsorption Experiments
The adsorption of PAC-IBU and removal efficiency by the selected activated carbon examined utilizing the batch equilibrium method. Different amount of sorbent has been tested (20, 40, 60, 80 and 100 mg) then suspended in the IBU solution and shaken until equilibrium time reached. After filtration, the amount of adsorbed PAC-IBU molecules were calculated from the variation between the initial and final concentration (Non-adsorbed molecules) of ibuprofen, measured by UV–visible spectrophotometer at a wavelength of 222.8 nm.7,13,22 On the other hand, in order to study the effect of temperature on the adsorption process the batch operated at different temperatures, also various solution pH have been conducted (pH= 3-12). The uptake of PAC-IBU per unit mass of sorbent was then determined through mass-balance calculations.11,20
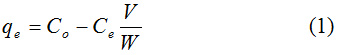
Where qe = is the amount of adsorbed PAC-IBU (mg/g), Co = Initial concentration (mgl-1), Ce = equilibrium concentration (mgl-1), W = Mass of adsorbent (mg) and V = Solution volume (ml). However, the removal percentage was determined using the equation below (Yaneva and Koumanova, 2006):
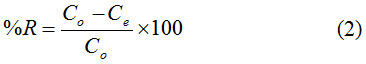
Where Co and Ce= Initial and final PAC-IBU concentration (mgl-1), R% = removal percentage. Meanwhile, scanning electron microscopy (SEM- model HTCE 3000g 0.01g), FT-IR (SHIMADZU), XRD (Rigaku Mini) have been applied for the loaded IBU for better understanding the adsorption process.
Results and Discussion
Characterization
The characteristic of FT-IR spectra which providing the essential information about the adsorption of PAC-IBU onto selected activated carbon. On the other hand, an intensity band was increased presenting maxima at 1012.99cm-1 and 1384.52 cm-1 assigned to C-O stretching in ether or ester groups due to bleaching.17,26 In accordance of Fig.1 (a and b) the bleaching treatment a new broad band present in the 3200 cm-1 and 3600 cm-1 due to the O-H stretching vibration of chemisorbed water and phenolic group.22 In addition, the peak centered at 1639.17 cm-1 only noticed after loading of PAC-IBU is assigned to the C=O stretching vibration of ester, ketone and/or carboxylic group. Finally, all spectra observed at 1580 cm-1 and 1618 cm-1assigned to C=C stretching formulizations of aromatic rings or C=O stretching vibration conjugated with aromaticrings.18
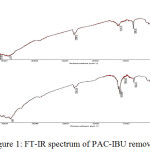 |
Figure 1: FT-IR spectrum of PAC-IBU removal. |
The XRD pattern Fig. 2 showed broad diffraction peak which is centered at 2θ = 21°and 2θ= 42°C. This illustrated that the medicine product-based activated carbon (MP-AC) was preponderantly amorphous as the presence of two broad Bragg were clearly demonstrated that the MP-AC is turbostratic structure and amorphous.23,27 It has clearly seen from Fig. 2a the diffraction does not have any sharp peaks throughout the range of 2-theta, hence proposed that MP-AC does not possess a crystalline nature.19 Nevertheless, amorphous-like structure with broad peak is necessary to examine the hybridization of the electrons of an atom of carbon Raman spectroscopy.10
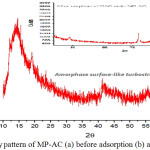 |
Figure 2: X-ray pattern of MP-AC (a) before adsorption (b) after adsorption. |
The broadened peak at 2θ= 42°C might be with the increasing temperature also increase in the intensity of the amorphous halo peak. Besides, maximum becomes more pronounced and moves to 43°C-44°C, which is characteristic of graphite and associated with the processes of graphitization of the organic-components and the formation of the nanocrystalline-structure of the matrix.14,31 Meanwhile, Fig. 3b. showed the diffractograms after the sorption process which easily understood that the adsorbate molecules successfully loaded onto the amorphous- like graphite sorbent surface. On the other hand, in both vividly recommended that the sorption system uniquely results change in the surface structure of the sorbent. Finally, the analyzed pattern of X-ray diffraction was also confirmed elsewhere.12,25
SEM-Morphology
The topographical information and surface structure of selected activated carbon after sorption process were achieved utilizing scanning electron microscopy (model NORAN) at an electron acceleration voltage of 25kV. The sample coated by a thin layer-gold before scanning to make conductive using splutter coater. Fig.3a showed the porous surface of the sorbent involved large holes, cracks, channels and full of cavities,2,27 mostly irregular with some smooth surfaces also can be observed. Whereas, Fig. 3b. elucidate the successful loaded of the adsorbate molecules which well-diffused in the pores of the sorbent surface then were aggregated-well via natural entrapped and governed by electrostatic force. As a result, it has easily visualized the dark spots, which can demarcate as a sign for efficient sorption system. In other words, the morphological study confirmed highly heterogeneous attached in the dark holes with various-classes of PAC molecules.21
 |
Figure 3: SEM-micrograph of (a) before sorption, (b) after sorption of PAC onto MP-AC. |
Optimization of Operation Parameters in A Batch System
Effect of Contact Time
One of the significant key factor for pharmaceuticals removal is study of contact time, the sorption process conducted at different contact times using selected activated carbon. Nevertheless, the adsorption of PAC-IBU rapidly increased with the raise of contact time at initial stages, on the other hand, gradually approached until reached equilibrium. Meanwhile, the rapid kinetics of the sorption process at initial stages perhaps attributed to high availability of binding sites.24,25,26 However, chemical attractions diffusion and other driving forces may take place. Hence, the adsorption rate decreased due to less porous as a result of the migration of pharmaceutical product. In addition, the adsorption efficiency reached maximum removal at 90 min (89.53%) and adsorption capacity was 36.72(mg/g). Also, after 120 min, the rate of removal of pharmaceuticals is constant indicate the equilibrium and the non-availability of sorption sites. The maximum uptake of drugs onto MP-AC observed at 90 min.32
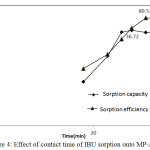 |
Figure 4: Effect of contact time of IBU sorption onto MP-AC. |
Effect of pH on the Removal Process
The pH of the solution is one of the important factors in controlling the adsorption process and particularly on the amount of adsorbed molecules by controlling the surface charge of the sorbent and by this way shows adsorbate-adsorbent electrostatic interaction (Oke et al., 2008). The obtained data showed highest amount of adsorbed molecules (qe=29.35mg/g) at pH 4, Fig.5 confirmed that as solution of pH increased to 12, adsorption capacity gradually decreased reaching minimums value at higher pH.
Thus, the ionization of PAC-IBU affected by solution pH based on its ionization pKa= 4.9, its expected that 50% of IBU become deprotonated at higher pH when (pH>pKa) similar data reported elsewhere.8,25
Overall, the fully or partially deprotonated surface charge due to an increase of pH, therefore a loss of positive charge may build up of negative charge.17 Also, as shown in Fig. 6 the point of zero charge (pHpzc) has been determined according to methods that reported from litterateurs and was found to be 6.68.23
At the time adsorption occurs at pH 9 (pH>pKa) the anionic form of PAC-IBU is dominant in solution and besides surface charge on the activated carbon is negatively charge when the solution pH is greater than pHpzc. Hence, the adsorption capacity is reduced due to electrostatic repulsion between the net surface charge of MP-AC and deprotonated PAC-IBU. In addition, at lower pH value, the net surface charge on MP-AC is positive and PAC-IBU is essentially non-dissociated. Consequently, the adsorption were enhanced due to the fact of repulsive electrostatic interactions are reduced.23,27
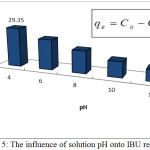 |
Figure 5: The influence of solution pH onto IBU removal. |
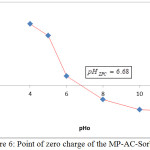 |
Figure 6: Point of zero charge of the MP-AC-Sorbent. |
Modeling of sorption isotherm
In this work various isotherm models were utilized for PAC-IBU removal from aquatic media and/or to relize the mechanism of the sorption system from physico-chemical view. Hence, constants of isotherms are commonly used in designing of the equilibrium data based on the hybird isotherm incoorporates three parameters into one emprical equation by combining both Freundlich and Langmuir models resulted mixed isotherm model as known by Redlich-Peterson model.24 The mathematical expression of the cominations are listed in equations 3and 4. Additionally, the sorption mechanism is a mix and does not follow ideal monolayer adsorption.
Freundlich-Langmuir equation:
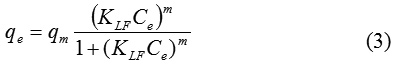
Redlich -Peterson equation:

However, in this work the Chi-square test has been evaluated using the mathematical expression below2,18:
where p denotes the number of experimental data, qcalc is calculated equilibrium concentration, qmeas is measured equilibrium concentration and q is the average of qcalc. The feature of utilizing the Chi-square test was the comparison of all isotherms on the same Abscissa and ordinate.19 When the measuring data from the model were similar to the experimental data, X2 would be a small number or vice versa. Moreover, the equilibrium data and constants of the above models are tabulated in Table 2.
Table 2: Constants and parameters of isotherm modeling obtained by calculation of the liner form.
| Isotherm |
Constants and Prameter,s |
|||||||
| Models | AR | KR | β | KF | 1/n | Qm | KL | X2 |
| Redlich-Peterson | 48.3071 | 0.5940 |
24.007 |
– | – | – | – | 0.01905 |
| Freundlich | – | – | – | 1.8693 | 0.17558 | – | – | 0.11837 |
| Langmuir | – | – | – | – | – | 23.4907 | 0.3782 | 1.49170 |
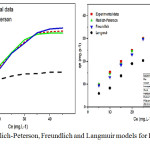 |
Figure 7: Redlich-Peterson, Freundlich and Langmuir models for PAC-IBU removal. |
Table 3: The coefficient of determination for the isotherm models.
|
Prarameter |
Value |
Fixed |
Error |
R2 |
| Experimental Data | ||||
| Intercept |
8.55889 |
◻ |
1.87447 |
0.94993 |
| Slope |
0.76773 |
◻ |
0.06662 |
|
| Redlich-Peterson | ||||
| Intercept |
8.16889 |
◻ |
2.08468 |
0.93986 |
| Slope |
0.77493 |
◻ |
0.07409 |
|
| Freundlich | ||||
| Intercept |
6.25667 |
◻ |
1.68331 |
0.9657 |
| Slope |
0.83987 |
◻ |
0.05983 |
|
| Langmuir | ||||
| Intercept |
6.92944 |
◻ |
2.11638 |
0.80857 |
| Slope |
0.409 |
◻ |
0.07522 |
|
Meanwhile, from Fig. 7 and Table 3. observed that the sorption capacity of PAC-IBU utlized MP-AC explained well enough by both Freunflich and Redlich-Peterson models which have highest coefficient of determination R2=0.9657 and R2= 0.9398 respectively. This might be availability of hetrogeneous surface with several classes of the sorbent-porosity caused physically holding of IBU molecules from the liquid phase.24,32 Also, Table2 revealed that the values of calcutaing small values of X2 and constants regarding to the sorption intensity (n) which is smaller than 1(1/n=0.17558) that both freundlich and Redlich-Peterson confirmed that the MP-AC has high capaibility for PAC-IBU removal within physically interactions.18,19 In addition, the linearized Langmuir shows the higher valeus of the X2 (1.49170) and lower R2 (0.8085) which indicated that the Langmuir isotherm is not approparate model to use.
Evaluation of Thermodynamic Parameters
In order to evaluate the thermodynamic parameters and effect of temperature as well on the sorption process, the sorption experiments were achieved at varied temperatures (298, 303, 313 and 323) K. As can be seen from Fig. 8 the raise of temperature affect negatively on the amount of qe (mg/g) and the percent of removal which decrease from 89.3% at 298K to 49.07% at 323K. Additionally, as temperature increases the sorption capacity decreases (37.02 to 18.79mg.g-1) obviously.12,14
Here, we observed that the increment of temperature certainty caused the destabilization of the physically binding forces and this clearly understood there is an ideal value to encourage the sorption of pharmaceutical products.
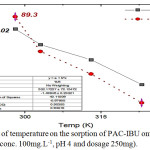 |
Figure 8: Effect of temperature on the sorption of PAC-IBU onto MP-AC (Initial conc. 100mg.L-1, pH 4 and dosage 250mg).Click here to view figure |
However, in order to measure the heat of sorption, which is the specification, that distinguishes the physisorption and chemisorption thermal process. In addition, heat of adsorption elucidated by Gibbs-Helmholtz.2
ΔG°= ΔH° – TΔS° (6)
ΔG°= – RT ln kc (7)
The Enthalpy change (∆H°) and Entropy change (∆S°) (i.e. heat of adsorption) are related with the Gibbs free energy by the equation.
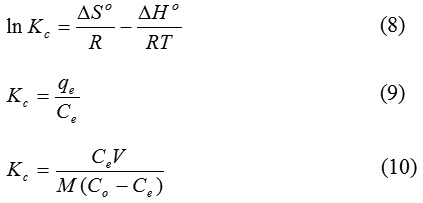
Where, Kc equilibrium constant, ∆Go Gibbs free energy (J mol-1), ∆So entropy (KJ mol-1 K-1), ∆Ho enthalpy (KJ mol-1), T absolute temperature (K), Co initial concentration of the IBU, Ce equilibrium concentration of the PAC-IBU, V volume of solution, M mass of the MP-AC, R gas constant (8.314 J mol-1 K-1).
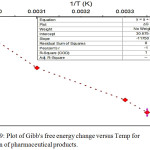 |
Figure 9: Plot of Gibb’s free energy change versus Temp for sorption of pharmaceutical products. |
In conductance of Fig.9, the negative value ∆Go confirmed the feasibility and spontaneous sorption process. Further, the entropy ∆So sorption and the heat of sorption ∆Ho on MP-AC were determined graphically from slop and intercept in a function of the inverse of the temperature in K of the medium.6,20 As can be seen from Table 4, the positive value of ∆Ho 7.94 kJ mol-1 indicates that the adsorption of PAC-IBU on the selected activated carbon is an endothermic process. Meanwhile, the positive value of ∆So0.028 kJ mol-1 K-1 agreed to an increment in randomness at the solid-interface with some structural changes in the adsorbent and adsorbate.1,15
Finally, in this work the ∆Go values are in the range of-3.6 to -9.02 kJ mol-1 confirming that the sorption of drug is mainly physical process.
Table 4: Iso Gibbs free energy change, enthalpy heat of sorption of pharmaceutical active products.
|
∆So (kJ mol-1 K-1) |
∆Ho (kJ mol-1) |
∆Go (kJ mol-1) |
|||
|
0.028 |
7.94 |
298K |
303K |
313K |
323K |
|
-9.02 |
-8.1 |
-5.75 |
-3.6 |
||
Conclusion
In the present study, new model approaches have been examined the sorption of pharmaceutical active products in aqueous solutions. Besides the acid pH solutions encouraged the sorption process achieved by electrostatic-interactions and the maximum uptake observed at pH4 (percentageR=89.3). In accordance of the equilibrium data the Redlich- Peterson model showed the best fit within lowest Chi-square test (X2=0.019) followed by Freundlich model (X2=0.11). In turn, the SEM micrographs confirmed that the sorption process successfully loaded which shows the heterogeneous surface covered by adsorbate molecules with several classes of sorbent-porosity. Besides, the XRD-analysis confirmed that the MP-AC preponderantly was turbostratic, in accordance to the range of 2-theta which proposed that the sorbent does not have a crystalline nature. On the other hand, varied thermodynamic parameters have also been examined and found that the sorption process was endothermic, feasible and spontaneous in nature, the increment of randomness at the solid-interface has confirmed by the value of entropy (∆So = 0.028 kJ mol-1 K-1). Finally, the information that gained from this work could help for giving remarkable details on the transport and maintenance of pharmaceutical active compounds in subsurface environment.
References
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M. and Blánquez, P., Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product, Chemical engineering journal. 2012; 211, pp.310-317.
- Rashid, B. Z.; Omar, R. A. & Salih, S. J., Characterization and Antimicrobial Efficiency of Silver Nanoparticles Based Reduction Method, International Journal of Current Microbiology and Applied Sciences. 2016; 5(8), 802-810.
- Bercu, J. P.; Parke, N. J.; Fiori, J. M. & Meyerhoff, R. D., Human health risk assessments for three neuropharmaceutical compounds in surface waters, Regulatory Toxicology and Pharmacology. 2008; 50, 420-427.
- Carrott, P. & Carrott, M. R., Lignin–from natural adsorbent to activated carbon: a review, Bioresource technology. 2007; 98, 2301-2312.
- Cermola, F.; Dellagreca, M.; Iesce, M. R.; Montella, S.; Pollio, A. & Temussi, F., A mild photochemical approach to the degradation of phenols from olive oil mill wastewater, Chemosphere. 2004; 55, 1035-1041.
- El-Rahman, K. A.; El-Kamash, A.; El-Sourougy, M. & Abdel-Moniem, N., Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A, Journal of radioanalytical and nuclear chemistry. 2006; 268, 221-230.
- Faraj, H. R. & Salih, S. J., Potential of Pistachio-Hard Shell Based Thiosemicarbazone-Acetophenone for Pb2+Metal Sorption: Kinetic Studies, Isotherms Modeling and Optimization, Journal of zankoy slemany. 2017; 19-1, 133-148.
- Hernando, M.; Mezcua, M.; Fernández-Alba, A. & Barceló, D., Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments, Talanta. 2006; 69, 334-342.
- Gazi, M. and Shahmohammadi, S., Removal of trace boron from aqueous solution using iminobis-(propylene glycol) modified chitosan beads, Reactive and Functional Polymers. 2012; 72(10), pp.680-686.
- Guedidi, H.; Reinert, L.; Soneda, Y.; Bellakhal, N. and Duclaux, L., Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths, Arabian Journal of Chemistry. 2017; 10, pp.S3584-S3594.
- Kyzas, G. Z.; Koltsakidou, A.; Nanaki, S. G.; Bikiaris, D. N. & Lambropoulou, D. A., Removal of beta-blockers from aqueous media by adsorption onto graphene oxide, Science of the Total Environment. 2015; 537, 411-420.
- Lindqvist, N.; Tuhkanen, T. & Kronberg, L., Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters, Water Research. 2005; 39, 2219-2228.
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; De Alencastro, L. F.; Abegglen, C.; Thonney, D.; Chèvre, N. & Schärer, M., Treatment of micropollutants in municipal wastewater: ozone or powdered activated carbon? Science of the total environment. 2013; 461, 480-498.
- Mittal, A.; Jhare, D. & Mittal, J., Adsorption of hazardous dye Eosin Yellow from aqueous solution onto waste material De-oiled Soya: Isotherm, kinetics and bulk removal, Journal of Molecular Liquids. 2013; 179, 133-140.
- Nanaki, S. G.; Kyzas, G. Z.; Tzereme, A.; Papageorgiou, M.; Kostoglou, M.; Bikiaris, D. N. & Lambropoulou, D. A., Synthesis and characterization of modified carrageenan microparticles for the removal of pharmaceuticals from aqueous solutions, Colloids and Surfaces B: Biointerfaces. 2015; 127, 256-265.
- Nowicki, P.; Kuszyńska, I.; Przepiórski, J. & Pietrzak, R., The effect of chemical activation method on properties of activated carbons obtained from pine cones, Central European Journal of Chemistry. 2013; 11, 78-85.
- Oke, I.; Olarinoye, N. & Adewusi, S., Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell, Adsorption. 2008; 14, 73-83.
- Oladipo, A.A. and Gazi, M., Fixed-bed column sorption of borate onto pomegranate seed powder-PVA beads: a response surface methodology approach, Toxicological & Environmental Chemistry. 2014; 96(6), pp.837-848.
- Olorundare, O.; Krause, R.; Okonkwo, J. & Mamba, B., Potential application of activated carbon from maize tassel for the removal of heavy metals in water, Physics and Chemistry of the Earth, Parts A/B/C. 2012; 50, 104-110.
- Ranjbar, M., Malakooti, E. and Sheshmani, S., Synthesis and characterization of mercury (II) complexes containing 2, 9-dimethyl-1, 10-phenanthroline by sonochemical method, Journal of Chemistry. 2012; 2013.
- Ra, J. S.; Oh, S.-Y.; Lee, B. C. & Kim, S. D., The effect of suspended particles coated by humic acid on the toxicity of pharmaceuticals, estrogens, and phenolic compounds, Environment international. 2008; 34, 184-192.
- Rivera-Jiménez, S. M.; Méndez-González, S. & Hernández-Maldonado, A., Metal (M= Co2+, Ni2+, and Cu2+) grafted mesoporous SBA-15: Effect of transition metal incorporation and pH conditions on the adsorption of Naproxen from water, Microporous and Mesoporous Materials. 2010;, 132, 470-479.
- Saber-Samandari, S.; Saber-Samandari, S. & Gazi, M., Cellulose-graft-polyacrylamide/hydroxyapatite composite hydrogel with possible application in removal of Cu (II) ions, Reactive and Functional Polymers. 2013; 73, 1523-1530.
- Salih, S. J., Removal of Basic Dyes from Aqueous Solution by Chloroacetic Acid Modified Ferula Communis Based Adsorbent: Thermodynamic and Kinetic Studies, Eastern Mediterranean University (EMU)-Doğu Akdeniz Üniversitesi (DAÜ). 2014.
- Salih, S. J.; Anwer, S. S. & Faraj, R. H., A Biosorption of Mercury from Wastewater Using Isolated Aspergillus Sp. Modified 1, 10-Phenanthroline: Hill Isotherm Model, Science Journal of University of Zakho. 2017; 5, 288-295.
- Salih, S. J. & Rashid, B., Cranberry Stem as an Efficient Adsorbent and Eco-Friendly for Removal of Toxic Dyes from Industrial Wastewater. Physico Studies, International Journal of Pharmaceutical Chemistry. 2015; 5, 207-17.
- Salih, S. J. & Smail, A. K., Synthesis, characterization and evaluation of antibacterial efficacy of zinc oxide nanoparticles, Pharmaceutical and biological evaluations. 2016; 3, 327-333.
- Snyder, S. A.; Adham, S.; Redding, A. M.; Cannon, F. S.; Decarolis, J.; Oppenheimer, J.; Wert, E. C. & Yoon, Y., Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals, Desalination. 2007; 202, 156-181.
- Song, Z. Y.; Xiao, W.; Hao, J.; Cui, Y. Z.; Lv, L. H. & Min, B. G., Kinetics and Thermodynamics of Sodium Alginate/Hydroxyapatite Composite Adsorption Fiber for Cd (II) Adsorping. Materials Science Forum, Trans Tech Publ. 2013; 578-583.
- Ternes, T. A.; Meisenheimer, M.; Mcdowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U. & Zulei-Seibert, N., Removal of pharmaceuticals during drinking water treatment, Environmental science & technology. 2002; 36, 3855-3863.
- Winker, M.; Faika, D.; Gulyas, H. & Otterpohl, R., A comparison of human pharmaceutical concentrations in raw municipal wastewater and yellowwater, Science of the total environment. 2008; 399, 96-104.
- Yaneva, Z. & Koumanova, B., Comparative modelling of mono-and dinitrophenols sorption on yellow bentonite from aqueous solutions, Journal of colloid and interface science. 2006; 293, 303-311.

This work is licensed under a Creative Commons Attribution 4.0 International License.









