A Novel Synthetic Route of Fused Tricyclic Framework Quinoline Derivatives from Readily Available Aliphatic Amino Carboxylic Acid Substrates
Department of Chemistry, Faculty of Science, University of Zakho, Kir-Iraq.
Corresponding Author E-mail: shireen2222@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350215
Article Received on : 21-11-2018
Article Accepted on : 14-03-2019
Article Published : 12 Apr 2019
A novel and an efficient strategy of fused tricyclic quinoline heterocycle compounds from aliphatic amino carboxylic acid substrates is disclosed. The protocol here is proceed over main reaction processes including: cyclization, protection, amidine formation, further cyclization and finally coupling with boronic acid substrate through Suzuki reaction. These reactions afforded the corresponding products in high yields. Furthermore, all synthesized compounds were identified by spectral data.
KEYWORDS:Aliphatic Amino Carboxylic Acid; Cross-Coupling Process; Cyclization; Diazotization; Nosylation Reactions, Palladium Catalyst; Protection Reactions; Suzuki Reaction
Download this article as:| Copy the following to cite this article: Mohammed S. A Novel Synthetic Route of Fused Tricyclic Framework Quinoline Derivatives from Readily Available Aliphatic Amino Carboxylic Acid Substrates. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Mohammed S. A Novel Synthetic Route of Fused Tricyclic Framework Quinoline Derivatives from Readily Available Aliphatic Amino Carboxylic Acid Substrates. Orient J Chem 2019;35(2). Available from: https://bit.ly/2UC9wPf |
Introduction
The heterocyclic compounds have a major interest role in medicinal chemistry,1 mild molecules furnishing with variety substituted functional groups consider to get much attention, due to their potential to design multiple chemical library. Meanwhile, nitrogen inclusive heterocycles such as quinolines propose specific advantage because of their different coordination of biological activities. For example, quinoline compounds were reported to control broad spectrum of beneficial biological activities universal anti-tuberculosis,2 antiproliferative3 anthelmintic,4 antibacterial,5 antioxidant activities6 antimalarial,7 inhibitors of oncogenic Ras,8 antitumor,9 antimicrobial10 anti-neoplastic,11 anti-oxidant 12 activity and anti-viral.13 In 1834, the first extraction of quinoline from coal tar was reported by Friedlieb Ferdinand Runge.14
Hence, because of these reasons plentiful attentiveness is paid for the synthesis and the biological value of quinoline nucleus, our current work is an amplification of our outstanding efforts toward design and developed novel and facile protocol towered synthesis of new fused tricyclic quinoline derivatives from readily available aliphatic amino carboxylic acid. Stable tricyclic heterocycle product compounds yields were obtained over several reaction processes, started with cyclization15 and protection of aliphatic amino carboxylic acid substrates, and followed by amidine formation,16 further cyclization,17 diazotization,18 nosylation19 and finally coupling with boronic acid substrate through Suzuki reaction process20 (Scheme 1).
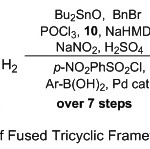 |
Scheme 1: Synthesis Route of Fused Tricyclic Framework Quinolines Substituents. |
Result and Discussion
In these precursory studies, we concentrated our effort on the construction of the fused tricyclic quinoline moiety starting from the readily available aliphatic amino carboxylic acid substrates (1-3). The planning (Scheme 2) of these reactions started with an effective the amide bond formation through an intramolecular cyclization reaction in the presence of Bu2SnO under refluxing toluene, using a Dean-Stark apparatus.21 While, a simple procedure for protection of amino groups (4-6) with benzyl group was applied under basic conditions to give the expected products (7-9) with high yields. Subsequently, the coupling of protected lactams (4-6) with 2-(trifluoromethyl)aniline 10 in presence of POCl3 reagent22 via Friedlander reaction should allow an access to an amidine intermediate (11-13). Furthermore, cyclization of the latter produced an aromatic amine (14-16), which under diazotization reaction conditions afforded the corresponding phenol products (17-19) in high yields. Conversion the phenol to the corresponding nosylate compounds (21-23), through reaction with p-nitrosulfonyl chloride 20, was reported to be a quite functional process. Finally, palladium-catalyzed cross-coupling23 of nosylate compounds (21-23) with aryl boronic acid substrate 23 and employing Suzuki reaction, provided the required products (24-26) very good yields.
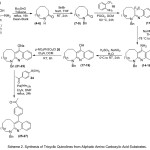 |
Scheme 2: Synthesis of Tricyclic Quinolines from Aliphatic Amino Carboxylic Acid Substrates. |
Generally, the proposed mechanism (Scheme 3) of preparation of lactams in the presence of Bu2SnO under refluxing toluene proceeds initial with stannylation of aliphatic amino carboxylic acid substrates (1-3) at the carboxylic acid group (1-3)i. This awards the way to the formation of a hydroxystannylene amino carboxylate intermediate (1-3)ii, which can undergo intramolecular cyclization to produce the desired lactam products (4-6) with removal of water molecule.
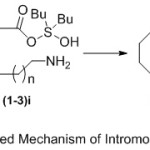 |
Scheme 3: Proposed Mechanism of Intromolecular Cyclization. |
Therefore, the suggested mechanism for the qualified cyclization of amidines is outlined in (Scheme 4). The first proton abstraction from (11-13) compounds in presence of base, lead to form the stable anion intermediate at -78°C. While increasing the temperature, it undergoes elimination of fluoride anion and give the quinone methide intermediate species. Further generation of anion in intermediates with the base, eliminate the second equivalent of fluoride. Finally, the resultant can undergo aromatization to afford the expected products (14-16) with the losing HF molecule.17
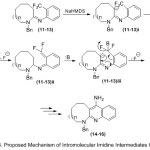 |
Scheme 4: Proposed Mechanism of Intromolecular lmidine Intermediates Cyclization. |
Experimental Part
Melting points were registered uncorrected 1H- and 13C-NMR spectra were obtained with Bruker DPX-300 FT-NMR spectrometer. The NMR spectra sitting in this article were measured in deuterium chloroform solution. IR spectra were reported with a Perkin-Elmer 1710-FTIR spectrometer. Revelation was carried out UV light at 254-365 nm. The purification was done through the flash chromatography process using Merck Silica Gel.
General Protocol for Synthesis of Lactams
An amino acids (0.63 mmol, 1 eq.) and di-n-butyloxide (156.8 mg, 0.63 mmol, 1 eq.) were stirred in refluxing toluene (120 mL) for 16 hours with use of a Dean-Stark system for the continuous distilling out the water. The solvent evaporated in vacuo at room temperature and the dissolved in CHCl3 (25 mL) and passed via short layer of Celite. The resultant was concentrated under vacuum and purified through the chromatography to produce the products (4-6) as a white solid.
azocan-2-one (4): (81%). (83-85)°C. IR νmax(cm-1) = 3320, 1685, 1645, 1465, 1331, 790. 1H NMR: δ (ppm) = 6.10 (s, 1H), 3.54 (apppearant t, 2H, J = 3 and 6 Hz, CH2), 2.93 (t, 2H, J = 3 Hz, CH2), 2.02-1.96 (m, 4H, 2CH2), 1.74-1.66 (m, 4H, 2CH2). 13C NMR: δ (ppm) = 175.9 (C=O), 41.7 (C), 37.3 (C), 29.1 (C), 28.7 (C), 27.2 (C), 24.9 (C).
azonan-2-one (5):(79%). (103-105)°C. IR νmax(cm-1) = 3325, 1681, 1540, 1330, 795. 1H NMR: δ (ppm) = 5.91 (s, 1H), 3.30 (t, 2H, J = 3 Hz, CH2), 2.84 (t, 2H, J = 3 Hz, CH2), 1.91-1.84 (m, 4H, 2CH2), 1.61-1.56 (m, 6H, 3CH2). 13C NMR: δ (ppm) = 175.9 (C=O), 41.1 (C), 37.7 (C), 30.0 (C), 29.5 (C), 29.5 (C), 28.0 (C), 25.7 (C).
azacyclododecan-2-one (6):(77%). (119-121)°C. IR νmax(cm–1) = 3324, 1686, 1645, 1466, 1332, 790. 1H NMR: δ (ppm) = 5.98 (s, 1H), 3.25 (t, 2H, J = 3 Hz, CH2), 2.44 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.87-1.81 (m, 4H, 2CH2), 1.58-1.52 (m, 12H, 6CH2). 13C NMR: δ (ppm) = 175.9 (C=O), 41.1 (C), 37.8 (C), 30.0 (C), 29.5 (3C), 29.5 (2C), 28.0 (C), 25.7 (C).
General Protocol for Synthesis of Benzyl Lactams
A solution of NaH (6.8 mg, 0.312 mmol, 1.5 eq.) in dry THF (10 mL) at 0°C, lactams (4-6) (0.205 mmol, 1 eq.) were added with stirring for 1 h. The ice removed and keep stirring for 24 hours at room temperature. The solvent removed under vacuum, followed by purification using flash chromatography to yield the products (7-9) as a pale yellow solid.
1-benzylazocan-2-one (7):(84%). (105-107)°C. IR νmax(cm-1) = 3070, 1950, 1810, 1666, 1451, 770. 1H NMR: δ (ppm) = 7.33-7.21 (m, 5H, CHar), 4.49 (s, 2H, CH2), 3.52 (t, 2H, J = 3 Hz, CH2), 2.86 (t, 2H, J = 6 Hz, CH2), 1.91-1.86 (m, 2H, CH2), 1.82-1.78 (m, 2H, CH2), 1.61-1.57 (m, 2H, CH2), 1.54-1.49 (m, 2H, CH2). 13C NMR: δ (ppm) = 176.5, 138.0, 128.8 (2), 128.6 (2), 127.7, 49.6, 47.2, 37.4 , 28.2, 27.0, 26.8, 24.8.
1-benzylazonan-2-one (8):(87%). (119-121)°C. IR νmax(cm-1) = 3071, 1952, 1814, 1665, 1451, 770. 1H NMR: δ (ppm) = 7.38-7.20 (m, 5H, CHar), 4.51 (s, 2H, CH2), 3.42 (t, 2H, J = 3 Hz, CH2), 2.41 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.83-1.77 (m, 4H, 2CH2), 1.55-1.49 (m, 6H, 3CH2). 13C NMR: δ (ppm) = 175.4, 137.9, 128.7 (2), 128.5 (2), 127.5, 49.4, 46.4, 36.8, 29.0, 29.0, 27.7, 27.0, 25.5.
1-benzylazacyclododecan-2-one (9):(79%). (123-125)°C. IR νmax(cm-1) = 3070, 1951, 1811, 1664, 1450, 770. 1H NMR: δ (ppm) = 7.31-7.19 (m, 5H, CHar), 4.48 (s, 2H, CH2), 3.43-4.36 (m, 2H, CH2), 2.38 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.81-1.73 (m, 4H, 2CH2), 1.52-1.46 (m, 12H, 6CH2). 13C NMR: δ (ppm) = 175.6, 138.1, 128.9 (2), 128.7 (2), 127.7, 49.9, 46.6, 37.0, 29.2 (3), 29.2 (2), 27.9, 27.8, 25.7.
General protocol for synthesis of Imidines: To benzyl lactams (7-9) (3.61 mmol, 1 eq.) in freshly distillated DCM (10 mL), POCl3 (0.66 mL, 7.2 mmol, 2 eq.) was added quietly. 2-(trifluoromethyl)aniline 10 (0.61 gm, 3.7 mmol, 1.05 eq.) in DCM was added quietly. The mixture was heating under reflux at 60°C for 24 hours. Ice-cold water was added followed by a 25% NaOH solution to pH 8. The extraction was done with DCM, brine, drying over MgSO4, the organic solvents were concentrated under vacuum and the resultant was purified by chromatography to yield the desired products (11-13) as yellow oil.
1-benzyl-N-(2-(trifluoromethyl)phenyl)azocan-2-imine (11):):(78%). IR νmax(cm-1) = 3071, 2942, 2217, 1580, 1377, 1237, 1088, 1205, 1020, 750, 730. 1H NMR: δ (ppm) = 7.64-7.63 (m, 1H, CHar), 7.43-7.24 (m, 8H, CH2), 4.68 (s, 2H, CH2), 3.71 (t, 1H, J = 3 Hz, CHa), 3.40 (t, 1H, J = 3 Hz, CHb), 2.81 (t, 2H, J = 3 Hz, CH2), 1.90-1.86 (m, 2H, CH2), 1.81-1.76 (m, 2H, CH2), 1.63-1.58 (m, 4H, 2CH2). 13C NMR: δ (ppm) = 145.2, 138.5, 134.3, 129.1 (2), 129.0 (2), 128.7, 128.1, 125.9, 125.2, 124.6, 123.1, 121.4, 52.0, 50.7, 32.6 , 28.7, 27.3, 27.2, 26.4.
1-benzyl-N-(2-(trifluoromethyl)phenyl)azonan-2-imine (12):(81%). IR νmax(cm-1) = 3072, 2941, 2219, 1584, 1378, 1237, 1088, 1206, 1020, 750, 730. 1H NMR: δ (ppm) = 7.65 (d, 1H, J = 6 Hz, CHar), 7.43-7.26 (m, 8H, CHar), 4.08 (s, 2H, CH2), 3.61 (t, 1H, J = 3 Hz, CHa), 3.52 (appearant t, 2H, J = 3 and 6 Hz, CHb), 2.52 (t, 2H, J = 3 Hz, CH2), 1.86-1.75 (m, 4H, 2CH2), 1.96-1.49 (m, 6H, 3CH2). 13C NMR: δ (ppm) = 176.4, 144.6, 138.1, 133.8, 128.6 (2), 128.4 (2), 127.6, 125.4, 124.7, 124.1, 122.6, 121.1, 51.5, 49.6, 33.3, 29.1, 29.0, 27.7, 27.6, 26.9.
1-benzyl-N-(2-(trifluoromethyl)phenyl)azacyclododecan-2-imine (13):(77%). IR νmax(cm-1) = 3070, 2941, 2217, 1580, 1378, 1237, 1089, 1204, 1020, 751, 730. 1H NMR: δ (ppm) = 7.58 (d, 1H, J = 6 Hz, CHar), 7.36-7.19 (m, 8H, CHar), 4.62 (s, 2H, CH2), 3.55-3.51 (m, 2H, CH2), 2.46 (t, 2H, J = 3 Hz, CHb), 1.80-1.69 (m, 4H, 2CH2), 1.50-1.43 (m, 12H, 6CH2). 13C NMR: δ (ppm) = 167.7, 144.9, 138.2, 134.0, 129.0 (2), 128.5 (2), 127.8, 125.6, 124.2, 122.9, 121.4, 121.1, 51.8, 49.9, 33.6, 29.4 (3), 29.3 (2), 28.1, 27.9, 27.2.
General Protocol for the Cyclization
To the imidines (11-13) (5.1 mmol, 1 eq.) in stirring dry THF were added to a vigorously solution of Sodium bis(trimethylsilyl)amide solution NaHMDS (4 molar eq.) at -78°C for 20 minutes. The reaction warmed slowly to -20°C for 1 hour, then at room temperature for 24 hours. The workup proceeded through quenched with brine then extraction with EtOAc (3Χ100 mL). The resultant transact over Na2SO4 then concentrated under vacuum. The purification via the flash chromatography, afforded the expected products (14-16) as red solid.
1-benzyl-1,2,3,4,5,6-hexahydroazocino[2,3-b]quinolin-7-amine (14): (83%). (134-136)°C. IR νmax(cm-1) = 3184, 2931, 1670, 1571, 1501, 1448, 1376, 1072, 1019, 735. 1H NMR: δ (ppm) = 8.07 (d, 1H, J = 3 Hz, CHar), 7.71 (dd, 1H, J = 3 and 6 Hz, CHar), 7.65-7.48 (m, 6H, CHar), 7.40-7.37 (m, 1H, CHar), 4.90 (s, 2H, CH2), 4.10 (t, 1H, J = 3 Hz, CHa), 3.81 (s, 2H, CH2), 3.62 (t, 1H, J = 3 Hz, CHb), 2.69 (t, 2H, J = 3 Hz, CH2), 2.04-2.00 (m, 2H, CH2), 1.94-1.89 (m, 2H, CH2), 1.70-1.66 (m, 2H, CH2). 13C NMR: δ (ppm) = 163.0, 153.5, 141.9, 137.9, 130.2, 130.0, 128.7 (2), 128.5 (2), 127.6, 124.6, 123.2, 115.5, 114.4, 52.9, 49.6, 29.0, 26.6, 26.2, 25.6.
1-benzyl-2,3,4,5,6,7-hexahydro-1H-azonino[2,3-b]quinolin-8-amine (15): (87%). (154-156)°C. IR νmax(cm-1) = 3175, 2931, 1672, 1570, 1501, 1448, 1376, 1072, 1019, 735. 1H NMR: δ (ppm) = 7.88 (dd, 1H, J = 3 and 6 Hz, CHar), 7.51 (dd, 1H, J = 3 and 6 Hz, CHar), 7.46-7.30 (m, 6H, CHar), 7.20-7.17 (m, 1H, CHar), 4.71 (s, 2H, CH2), 3.95 (s, 2H, CH2), 3.63-3.58 (m, 2H, CH2), 2.58 (t, 2H, J = 3 Hz, CH2), 1.96-1.91 (m, 2H, CH2), 1.86-1.80 (m, 2H, CH2), 1.61-1.55 (m, 4H, CH2). 13C NMR: δ (ppm) = 166.1, 153.7, 142.1, 138.0, 130.3, 130.0, 128.8 (2), 128.6 (2), 127.6, 124.8, 123.3, 115.7, 114.4, 53.1, 44.2, 30.0, 29.0, 27.7, 27.7, 25.8.
1-benzyl-1,2,3,4,5,6,7,8,9,10-decahydro-[1]azacyclododecino[2,3-b]quinolin-11-amine (16): (85%). (177-179)°C. IR νmax(cm-1) = 3178, 2931, 1670, 1571, 1509, 1450, 1376, 1072, 1020, 735. 1H NMR: δ (ppm) = 7.79 (d, 1H, J = 6 Hz, CHar), 7.46 (d, 1H, J = 3 Hz, CHar), 7.32-7.21 (m, 6H, CHar), 7.10-7.07 (m, 1H, CHar), 4.61 (s, 2H, CH2), 3.67 (s, 2H, CH2), 3.58 (appearant t, 1H, J = 3 and 6 Hz, CHa), 3.49 (t, 1H, J = 3 Hz, CHb), 2.49 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.66-1.61 (m, 2H, CH2), 1.76-1.73 (m, 2H, CH2), 1.53-1.46 (m, 10H, CH2). 13C NMR: δ (ppm) = 166.1, 156.1, 153.8, 142.0, 138.0, 130.3, 130.0, 128.8 (2), 128.6 (2), 127.6, 124.8, 123.3, 53.0, 49.0, 30.0, 29.2 (3), 29.0 (2), 27.7, 27.6, 25.8.
General Protocol for Preparation of Phenols
To the amines (14-16) (0.2 mmol, 1 eq.) and H2SO4 (53 µmL, 1 mmol, 5 eq.), NaNO2 (690 mg, 1 mmol, 5 eq.) in H2O (2.5 mL) was added dropwise at 0°C, and then stirring 24 hours at room temperature. A 25% NaOH solution was added quietly at 0°C to pH = 8-9. The product was extracted with EtOAc, brine, dried over MgSO4, then evaporation the solvent in vacuo. The purification through flash chromatography, gave the expected products (17-19) as a white solid.
1-benzyl-1,2,3,4,5,6-hexahydroazocino[2,3-b]quinolin-7-ol (17):(70%). (203-205) °C. IR νmax(cm-1) = 3340, 2975, 1620, 1386, 1499, 1473, 1090, 1050, 885, 742. 1H NMR: δ (ppm) = 7.82 (dd, 1H, J = 3 and 6 Hz, CHar), 7.71 (dd, 1H, J = 3 and 6 Hz, CHar), 7.37-7.15 (m, 7H, CHar), 4.62 (s, 2H, CH2), 3.62 (t, 1H, J = 3 Hz, CHa), 3.34 (appearant t, 1H, J = 3 and 6 Hz, CHb), 2.45 (t, 2H, J = 3 Hz, CH2), 1.75-1.72 (m, 2H, CH2), 1.66-1.61 (m, 2H, CH2), 1.42-1.37 (m, 3H, CH2). 13C NMR: δ (ppm) = 169.1, 163.8, 142.7, 138.2, 131.4, 129.0 (2), 128.8, 127.8, 127.9, 127.3, 125.2, 124.3, 122.6, 115.2, 53.3, 50.0, 29.3, 27.1, 26.1, 24.0.
1-benzyl-2,3,4,5,6,7-hexahydro-1H-azonino[2,3-b]quinolin-8-ol (18):(74%). (215-217) °C. IR νmax(cm-1) = 3342, 2975, 1621, 1389, 1450, 1473, 1090, 1050, 885, 742. 1H NMR: δ (ppm) = 7.81 (dd, 1H, J = 3 and 6 Hz, CHar), 7.62 (dd, 1H, J = 3 and 9 Hz, CHar), 7.35-7.14 (m, 7H, CHar), 4.61 (s, 2H, CH2), 3.52-3.49 (m, 2H, CH2), 2.53 (t, 2H, J = 3 Hz, CHb), 1.87-1.83 (m, 2H, CH2), 1.76-1.71 (m, 2H, CH2), 1.52-1.46 (m, 4H, 2CH2), 1.40 (s, 1H, OH). 13C NMR: δ (ppm) = 169.0, 167.1, 142.7, 138.3, 131.6, 129.1 (2), 129.0 (2), 128.1, 127.5, 125.3, 124.5, 122.7, 115.5, 53.3, 49.4, 30.3, 29.2, 28.1, 28.1, 23.7.
1-benzyl-1,2,3,4,5,6,7,8,9,10-decahydro-[1]azacyclododecino[2,3-b]quinolin-11-ol (19): (73%). (235-237) °C. IR νmax(cm-1) = 3340, 2975, 1620, 1386, 1499, 1479, 1095, 1047, 885, 742. 1H NMR: δ (ppm) = 7.86 (d, 1H, J = 6 Hz, CHar), 7.66 (s, 1H, CHar), 7.28-7.14 (m, 7H, CHar), 4.58 (s, 2H, CH2), 3.51-3.46 (m, 2H, CH2), 2.51 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.86-1.79 (m, 2H, CH2), 1.74-1.69 (m, 2H, CH2), 1.51-1.44 (m, 10H, 5CH2). 13C NMR: δ (ppm) = 168.7, 166.3, 142.2, 137.8, 128.6 (2), 128.3 (2), 127.5, 124.7, 123.9, 122.3, 115.5, 52.9, 49.9, 29.7, 29.1, 28.8 (4), 27.5 (3), 23.2.
General Procedure of Nosylations
To a solution of phenols (17-19) (0.2 mmol, 1 eq.) in freshly distillated DCM (2.2 mL), was added triethyl amine (20 µmL, 0.2 mmol, 1 eq.) with stirring 30 min at 0°C. Then p-nitrobenzene sulfonyl chloride 20(44 mg, 0.2 mmol, 1 eq.) in DCM was added quietly to the mixture of the reaction mixture then stirring for 6 hours at room temperature. The resultant was concentrated and dissolved in EtOAc, followed by washing with 1N HCl, 1N NaOH, saturated NaCl solution. The organic layer was removed by vacuum. The purifiecation through flash chromatography, produced the expected products (21-23) as yellow solid.
1-benzyl-1,2,3,4,5,6-hexahydroazocino[2,3-b]quinolin-7-yl 4-nitrobenzenesulfonate (21): (79%). (182-184) °C. IR (ATR) νmax (cm-1) = 2962, 1615, 1258, 1084, 1014, 791, 745. 1H NMR: δ (ppm) = 7.46 (d, 2H, J = 6 Hz, CHar), 8.29 (d, 2H, J = 6 Hz, CHar), 7.77 (dd, 1H, J = 3 and 6 Hz, CHar), 7.75 (d, 1H, J = 6 Hz, CHar), 7.29-7.16 (m, 7H, CHar), 4.61 (s, 2H, CH2), 3.62 (t, 1H, J = 3 Hz, CHa), 3.33 (t, 1H, J = 3 Hz, CHb), 2.46 (t, 2H, J = 3 Hz, CH2), 1.75-1.71 (m, 2H, CH2), 1.60-1.55 (m, 2H, CH2), 1.40-1.35 (m, 2H, CH2). 13C NMR: δ (ppm) = 164.9, 159.0, 147.5, 146.1, 145.0, 138.1, 132.1, 131.2, 130.6 (2), 129.0, 128.8 (2), 128.7 (2), 127.6 (2), 124.5, 124.2 (2), 123.6, 53.0, 49.7, 29.2, 26.8, 25.8, 24.1.
1-benzyl-2,3,4,5,6,7-hexahydro-1H-azonino[2,3-b]quinolin-8-yl 4-nitrobenzenesulfonate (22):(86%). (197-199) °C. IR (ATR) νmax (cm-1) = 2961, 1617, 1259, 1085, 1015, 794, 744. 1H NMR: δ (ppm) = 8.50 (d, 2H, J = 3 Hz, CHar), 8.24 (d, 2H, J = 6 Hz, CHar), 7.85 (d, 1H, J = 3 Hz, CHar), 7.57 (d, 1H, J = 6 Hz, CHar), 7.32-7.30 (m, 6H, CHar), 7.15-7.12 (m, 1H, CHar), 4.62 (s, 2H, CH2), 3.56-3.51 (m, 2H, CH2), 2.57 (t, 2H, J = 3 Hz, CH2), 1.88-1.84 (m, 2H, CH2), 1.77-1.72 (m, 2H, CH2), 1.53-1.48 (m, 4H, 2CH2). 13C NMR: δ (ppm) = 167.9, 159.0, 147.5, 145.1, 138.0, 132.1, 131.3, 130.6 (2), 129.1, 128.8 (2), 128.6 (2), 128.0, 127.8 (2), 124.5, 124.2, 123.5, 53.1, 49.2, 30.1, 29.1, 27.8 (2), 27.7, 23.7.
1-benzyl-1,2,3,4,5,6,7,8,9,10-decahydro-[1]azacyclododecino[2,3-b]quinolin-11-yl 4-nitrobenzenesulfonate (23): (89%). (210-212).
1-benzyl-1,2,3,4,5,6,7,8,9,10-decahydro-[1]azacyclododecino[2,3-b]quinolin-11-yl 4-nitrobenzenesulfonate (23): (89%). (210-212) °C. IR (ATR) νmax (cm-1) = 2959, 1619, 1259, 1084, 1012, 794, 735. 1H NMR: δ (ppm) = 8.49 (d, 2H, J = 6 Hz, CHar), 8.23 (d, 2H, J = 6 Hz, CHar), 7.85 (dd, 2H, J = 3 and 6 Hz, CHar), 7.30-7.19 (m, 7H, CHar), 4.61 (s, 2H, CH2), 3.53-3.50 (m, 2H, CH2), 2.58 (appearant t, 2H, J = 3 and 9 Hz, CH2), 1.87-1.82 (m, 2H, CH2), 1.77-1.71 (m, 2H, CH2), 1.52-1.46 (m, 10H, 3CH2). 13C NMR: δ (ppm) = 167.9, 159.1, 147.5, 146.1, 145.2, 138.1, 132.0, 131.4 (2), 130.6, 129.1, 128.9 (2), 128.7 (2), 128.1, 127.7, 124.2 (2), 123.5, 53.1, 49.2, 30.1, 29.2 (3), 29.0 (2), 27.7, 27.7, 23.6.
General Procedure for the Coupling Reaction
To the Nosylate compounds (21-23) (0.18 mmol, 1 eq.) in DMF (5 mL), Boronic acid substrate 24 (0.56 mmol, 2 eq.) and Et3N (30 mL, 0.2 mmol, 1.2 eq.) were added. The solution was bubbled for 30 minutes, then Pd-catalyst (21 mg, 0.018 mmol, 0.1 eq.) was added and reflux overnight. The solvent evaporated under vacuum. The purification of the crude mixture through chromatography, gave the pure products (25-27) as a white solid.
1-(4-(1-benzyl-1,2,3,4,5,6-hexahydroazocino[2,3-b]quinolin-7-yl)phenyl)ethan-1-one (25):(89%). (239-241) °C. IR (ATR) νmax (cm-1) = 3075, 2924, 1710, 1560, 1493, 1431, 1395, 1366, 1346, 1170, 741. 1H NMR: δ (ppm) = 8.01 (d, 2H, J = 6 Hz, CHar), 7.93 (d, 1H, J = 3 Hz, CHar), 7.73 (d, 2H, J = 3 Hz, CHar), 7.39 (d, 1H, J = 3 Hz, CHar), 7.31-7.27 (m, 4H, CHar), 7.25-7.19 (m, 2H, CHar), 7.12-7.09 (m, 1H, CHar), 4.62 (s, 2H, CH2), 3.82 (t, 1H, J = 3 Hz, CHa), 3.34 (appearant t, 1H, J = 3 and 6 Hz, CHb), 2.99 (s, 3H, CH3), 2.44 (t, 2H, J = 3 Hz, CH2), 1.75-1.59 (m, 2H, CH2), 1.63-1.59 (m, 2H, CH2), 1.41-1.36 (m, 2H, CH2). 13C NMR: δ (ppm) = 197.7, 163.0, 153.1, 144.5, 138.8, 137.9, 137.0, 135.7, 131.5, 130.1 (2), 129.1, 128.5 (2), 128.3, 128.1, 127.8 (2), 127.5 (2), 125.7, 124.3, 52.8, 49.5, 36.0, 29.0, 27.7, 26.6, 25.5.C. IR (ATR) νmax (cm-1) = 2959, 1619, 1259, 1084, 1012, 794, 735. 1H NMR: δ (ppm) = 8.49 (d, 2H, J = 6 Hz, CHar), 8.23 (d, 2H, J = 6 Hz, CHar), 7.85 (dd, 2H, J = 3 and 6 Hz, CHar), 7.30-7.19 (m, 7H, CHar), 4.61 (s, 2H, CH2), 3.53-3.50 (m, 2H, CH2), 2.58 (appearant t, 2H, J = 3 and 9 Hz, CH2), 1.87-1.82 (m, 2H, CH2), 1.77-1.71 (m, 2H, CH2), 1.52-1.46 (m, 10H, 3CH2). 13C NMR: δ (ppm) = 167.9, 159.1, 147.5, 146.1, 145.2, 138.1, 132.0, 131.4 (2), 130.6, 129.1, 128.9 (2), 128.7 (2), 128.1, 127.7, 124.2 (2), 123.5, 53.1, 49.2, 30.1, 29.2 (3), 29.0 (2), 27.7, 27.7, 23.6.
General Procedure for the Coupling Reaction
To the Nosylate compounds (21-23) (0.18 mmol, 1 eq.) in DMF (5 mL), Boronic acid substrate 24 (0.56 mmol, 2 eq.) and Et3N (30 mL, 0.2 mmol, 1.2 eq.) were added. The solution was bubbled for 30 minutes, then Pd-catalyst (21 mg, 0.018 mmol, 0.1 eq.) was added and reflux overnight. The solvent evaporated under vacuum. The purification of the crude mixture through chromatography, gave the pure products (25-27) as a white solid.
1-(4-(1-benzyl-1,2,3,4,5,6-hexahydroazocino[2,3-b]quinolin-7-yl)phenyl)ethan-1-one (25):(89%). (239-241) °C. IR (ATR) νmax (cm-1) = 3075, 2924, 1710, 1560, 1493, 1431, 1395, 1366, 1346, 1170, 741. 1H NMR: δ (ppm) = 8.01 (d, 2H, J = 6 Hz, CHar), 7.93 (d, 1H, J = 3 Hz, CHar), 7.73 (d, 2H, J = 3 Hz, CHar), 7.39 (d, 1H, J = 3 Hz, CHar), 7.31-7.27 (m, 4H, CHar), 7.25-7.19 (m, 2H, CHar), 7.12-7.09 (m, 1H, CHar), 4.62 (s, 2H, CH2), 3.82 (t, 1H, J = 3 Hz, CHa), 3.34 (appearant t, 1H, J = 3 and 6 Hz, CHb), 2.99 (s, 3H, CH3), 2.44 (t, 2H, J = 3 Hz, CH2), 1.75-1.59 (m, 2H, CH2), 1.63-1.59 (m, 2H, CH2), 1.41-1.36 (m, 2H, CH2). 13C NMR: δ (ppm) = 197.7, 163.0, 153.1, 144.5, 138.8, 137.9, 137.0, 135.7, 131.5, 130.1 (2), 129.1, 128.5 (2), 128.3, 128.1, 127.8 (2), 127.5 (2), 125.7, 124.3, 52.8, 49.5, 36.0, 29.0, 27.7, 26.6, 25.5.
1-(4-(1-benzyl-2,3,4,5,6,7-hexahydro-1H-azonino[2,3-b]quinolin-8-yl)phenyl)ethan-1-one (26): (85%). (230-232) °C. IR (ATR) νmax (cm-1) = 3074, 2924, 1711, 1560, 1490, 1429, 1390, 1366, 1346, 1170, 742. 1H NMR: δ (ppm) = 8.01 (d, 2H, J = 9 Hz, CHar), 7.93 (dd, 1H, J = 3 and 6 Hz, CHar), 7.73 (d, 2H, J = 3 Hz, CHar), 7.40 (d, 1H, J = 6 Hz, CHar), 7.32-7.11 (m, 7H, CHar), 4.62 (s, 2H, CH2), 3.56-3.51 (m, 2H, CH2), 2.99 (s, 3H, CH3), 2.53 (appearant t, 2H, J = 6 and 9 Hz, CH2), 1.88-1.83 (m, 2H, CH2), 1.77-1.72 (m, 2H, CH2), 1.53-1.48 (m, 4H, 2CH2). 13C NMR: δ (ppm) = 199.1, 167.8, 155.0, 146.3, 140.7, 139.7, 138.6, 137.6, 133.5, 131.8, 130.9, 130.5, 130.2 (2), 129.8, 129.8, 129.4, 127.5 (2), 126.2 (2), 54.7, 50.7, 36.5, 31.7, 30.7, 30.6, 29.6, 29.4, 29.3.
1-(4-(1-benzyl-1,2,3,4,5,6,7,8,9,10-decahydro-[1]azacyclododecino[2,3-b]quinolin-11-yl)phenyl)ethan-1-one (27):(81%). (251-253) °C. IR (ATR) νmax (cm-1) = 3075, 2925, 1711, 1560, 1493, 1431, 1395, 1370, 1346, 1170, 741.1H NMR: δ (ppm) = 7.99 (d, 2H, J = 3 Hz, CHar), 7.96 (dd, 1H, J = 3 and 6 Hz, CHar), 7.70 (d, 2H, J = 3 Hz, CHar), 7.39 (d, 1H, J = 3 Hz, CHar), 7.29-7.11 (m, 7H, CHar), 4.61 (s, 2H, CH2), 3.52 (t, 2H, J =3 Hz, CH2), 2.57 (s, 3H, CH3), 2.54 (appearant t, 2H, J = 3 and 6 Hz, CH2), 1.87-1.82 (m, 2H, CH2), 1.77-1.72 (m, 2H, CH2), 1.52-1.46 (m, 10H, 5CH2). 13C NMR: δ (ppm) = 199.0, 167.8, 155.1, 140.7, 139.7, 137.6 (2), 133.4 (2), 131.9 (2), 130.9, 130.4 (2), 129.8 (2), 129.4 (2), 127.4 (2), 126.1 (2), 54.7, 50.7, 36.6, 31.7, 30.8 (3), 30.6, 29.7, 29.4, 29.3.
Conclusion
In outline, we have presented a functional and useful across the construction of fused tricyclic heterocyclic quinolines. This method was developed and started first with the cyclization and protection of aliphatic amino carboxylic acids and then followed by the amidine formation through the Vlismeier intermediate. Moreover, the cyclization process with 2-(trifluoromethyl)aniline substrate, provided the amino compounds. Transformation of the latter to the corresponding hydroxyl then nosylate analogies, pliable the conclusive Suzuki reaction to proceed simply in the involvement of aromatic boronic acid substrate and affording the expected products in high yields.
Acknowledgements
Authors is grateful to the Faculty of Science, Chemistry Department, Zakho University for providing all facilities.
References
- (a) Cravotto, G., Nano, G.M., Palmisano, G., Tagliapietra, S., Tetrahedron Asymmetry, 2001, 12, 707-709; (b). Kayser, O., Kolodziej, H., Planta Med. 1997, 63, 508-510; (c) Wang, C.J., Hsieh, Y. J., Chu, C.Y., Lin, Y. L., Tseng, T. H., Cancer Lett. 2002, 183, 163-168; (d) Kirkiacharian, S., Thuy, D.T., Sicsic, S., Bakhchinian, R., Kurkjian, R., Tonnaire, T., Farmaco, 2002, 57, 703-708.
- (a) Puneet, P. J., Mariam, S. D, Archana, R., Muktikanta, R., Rajan, M. G., Rational drug design based synthesis of novelarylquinolines as anti-tuberculosis agents, Bioorg. Med. Chem. Lett. 2013, 23, 6097-6105; (b) Upadhayaya, R. S., Kulkarni, G. M., Vasireddy, N. R., Vandavasi, J. K., Dixit S. S., Sharma, V. et al. Design, synthesis and biologicalevaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis, Bioorg. Med. Chem. 2009, 17, 4681-4692
- (a) Yeh, L. C., Chao, J. H., Zun, Y. H., Chih, H. T., Feng, S. C., Sheng, H. Y. et alBioorg. Med. Chem. 2006, 14, 3098-3105; (b) Eun, J. K., Mohammed, I. E., Chang, H. O., So, H. L., Taebo, S., Garam, K. et al. Eur. J. Med. Chem. 2013, 70, 10-21.
- Sharon, R., Jean, M. P., Whitfield, P. J., Jonesa, K., Bioorg. Med. Chem. Let. 2005, 15, 4806-4808.
- Kidwai, M., Bhushan, K. R., Sapra, P., Saxena, R. K., Gupta, R., Bioorg. Med. Chem. 2000, 8, 69-72.
- Nuran, K., Busra, Y., Ayca, A., Zeynep, I., Fatih, S. B., Nurettin, Y., Eur. J. Med. Chem. 2013, 69, 348-355.
- (a) Curd, F. H.S, Raison, C. G., Rose, F. L,. J. Chem. Soc. 1947, 899-909; (b) Gemma, S., Kukreja, G., Fottorusso, C., Persico, M., Romano, M. P., Altarelli, M. et al. Bioorg. Med. Chem. Lett. 2006, 16, 5384-5388; (c) Mukesh, C. J., Kathryn, J. W., Dale, T., Roger, H., Peter, J. S., Timothy J. E., Eur. J. Med. Chem. 2013, 69, 338-347.
- (a) El-Sayed, O. A., Aboul-Enein, H. Y., Arch. Pharm. (Weinheim) 2001, 334, 117-120; (b) Wolin, R., Wang, D., Kelly, J., Afonso, A., James, L., Kirschmeier, P., Mcphail, A. T., Bioorg. Med. Chem. Lett. 1996, 6, 195- 200.
- Tsuzuki, Y., Yomita, K., Sato, Y., Bioorg. Med. Chem. Lett. 2004, 14, 1216.
- Antoine, M., Barreau, M., Descon, C., Philippe, G., Guy, P., US Pat. 2003, 6548506.
- Catoen, C., Facompre, M., Houssin, R., Pommery, N., Goossens, J., Colson, P. et al, J. Med. Chen. 2004, 47, 3665-3673.
- Aoblovich, M., Prenzler, P. D., Pabalides, E., McDonald, S., Robards, K., Analyst, 2002, 127, 183-198.
- Benard, C., Zoubiri, F., Nor, B. N., Danet, M., Desmele, D., Leh, H. et al., Bioorg. Med. Chem. Lett. 2004, 14, 2473- 2476.
- (a) Chisholm, H., Quinoline. Encyclopædia Britannica, 11th ed., Cambridge University, 759, 1911; (b) Runge, F. F., Annalen der Physik und Chemie, 1834, 31(5), 65-78.
- Tsui, S.-K.; Wood, J. D. Can. J. Chem. 1979, 57, 1977-1979.
- (a) Mohammed, S., Robert, F., Landais, Y., Development of new radical processes: approaches toward the synthesis of Eucophylline, thesis, 2014; (b) Hassan, H., Mohammed, S., Robert, F., Landais, Y., Org. Lett., 2015, 17(18), 4518-4521; (c) Mohammed, S., Maher, K., Indian J. Hetero. Chem., 2019, 29(1), 21-25.
- Smith, L., Kiselyov, A. S., Tetrahedron Lett. 1999, 40(31), 5643-5646.
- (a) Patai, S., Chemistry of the Diazonium and Diazo Groups: Part 1., Ed., Wiley-Blackwell, 1978; (b) Patai, S., Chemistry of the Diazonium and Diazo Groups: Part 2., Ed., Wiley-Blackwell, 1978.
- Ciattini, P. G.; Morera, E.; Ortar, G. Tetrahedron Lett. 1992, 33, 4815-4818.
- (a) Suzuki A., Pure & Appl. Chem. 1991, 63, 419; (b) Norio, M., Akira, S., Chemical Reviews, 1995, 95, 2457-2483.
- (a) Dean, E. W., Stark, D., D., The Journal of Industrial & Engineering Chemistry, 1960, 12(5), 486-490; (b) Wiberg, K., B., Laboratory Technique in Organic Chemistry. McGraw-Hill series in advanced chemistry. New York: McGraw Hill, 1960.
- (a) Mohammed, S., Maher, K., Indian J. Hetero. Chem. 2017, 27, 1-6; (b) Mohammed, S., Maher, K., Indian J. Hetero. Chem. 2017, 27, 83-87; (c) Mohammed, S., Khalid, M., Orient. J. Chem., 2015, 31(4), 2137-2146.
- (a) Miyaura, N., Yamada, K., Suzuki, A., Tetrahedron Lett. 1979, 20, 3437-3440; (b) Miyaura N. and Suzuki A., Chem. Comm., 1979, 19, 866-867; (c) Miyaura N. and Suzuki A., Chemical Reviews., 1995, 95, 2457-2483.

This work is licensed under a Creative Commons Attribution 4.0 International License.










