1, 4 Dithiane 2, 5 Diol : An Versatile Monomer to Synthesis Aliphatic Random Copolyester with Biomedical Application
Kalpana Balachandran*1 , Nanthini Raveendiran1 and Margaret Marie John2
, Nanthini Raveendiran1 and Margaret Marie John2
1Department of Chemistry, Pachaiyappa’s College, Chennai, India.
2Department of Chemistry, Women's Christian College, Chennai, India.
Corresponding Author E-mail: kalpanajanarthanan2005@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350253
Article Received on : 20-02-2019
Article Accepted on : 05-04-2019
Article Published : 29 Apr 2019
This article uses 1, 4-dithiane-2, 5-diol as a monomer to synthesize aliphatic random copolyester (PDDD).PDDD was synthesized by direct melt polycondensation method and characterized by FT-IR and 1H- NMR. The physical properties of PDDD were characterized by X-ray diffraction, differential scanning calorimetry, as well as viscosity and solubility measurements. The anticancer, antioxidant, and antimicrobial activity of PDDD were evaluated to investigate its potential biomedical applications. Generally, good results were obtained. It is evident that the copolyester exhibits favorable and tunable physical, thermal and biological properties and so is a suitable candidate for biomedical applications.
KEYWORDS:Anticancer; Antimicrobial; Antioxidant; Copolyester; 1,4 Dithiane 2,5 Diol
Download this article as:| Copy the following to cite this article: Balachandran K, Raveendiran N, John M. M. 1, 4 Dithiane 2, 5 Diol : An Versatile Monomer to Synthesis Aliphatic Random Copolyester with Biomedical Application. Orient J Chem 2019;35(2). |
| Copy the following to cite this URL: Balachandran K, Raveendiran N, John M. M. 1, 4 Dithiane 2, 5 Diol : An Versatile Monomer to Synthesis Aliphatic Random Copolyester with Biomedical Application. Orient J Chem 2019;35(2). Available from: https://bit.ly/2L7PA21 |
Introduction
Aliphatic copolyesters are the most economically competitive biodegradable polymers1 as they possess both biodegradability and biocompatibility together with interesting physical and chemical properties and finds immense application in biomedical field.2-7 In recent years, a lot of research efforts have been targeted to develop special high performance polymer with sulphur in backbone as they possess excellent properties and wide applications. The sulphur containing polymers are optically active, liquid crystalline, flame retardant and used as fuel cell materials8 and PHAs containing sulphur has wide application in medicine, pharmacy, packaging industry and agriculture.9 It was reported that sulphur containing plastics have high refractive index, high Abbe number, good impact strength, excellent machinability, good tintability and good transmittance.10 An exhaustive literature survey revealed that 1,4 dithiane 2,5 diol is the versatile monomer with sulphur atom that has special properties and excellent applications to synthesis of aliphatic random copolyester. The versatile monomer 1,4 dithiane 2,5 diol finds wide application in synthesizing optical material with high refractive index,11 food additive as flavoring agent, biocontrol agent against plant pathogen,12 adhesives,13 in producing polymerisable compositions14 and antireflective coatings.15 The 1,4 dithiane 2,5 diol is also used as monomer in polyurethane biomaterials that has been used as insulation on pacing leads,16 in synthesizing polymers for use in photoresist formulation for 193nm Immersion lithography17 and in light fast polyurethane composition that possess high light resistance, visual properties and high heat shape retention with wide application.18 It is evident that 1,4 dithiane 2,5 diol is biodegradable and environment friendly as it has been used as a source of sulphur for Mycobacterium phlei GTIS10 for microbial desulphurization which is essential study to remove the sulphur di oxide from combustion of fossil fuels.19 The versatile monomer 1,4 dithiane 2,5 diol was used in synthesis of thiophene derivative20 and 1,4 Thienodiazepine -2,5- diones which exhibited promising antagonistic activity against p53-Mdm2 interaction.21 The 1,4 dithiane derivative was used as NCP7 (nucleocapsid Protein) zinc finger targeted agent against retrovirus replication.22 Therefore the present research work is focused on the synthesis and characterization of new aliphatic Poly(1,4 dithiane 2,5 diol dodecanedioate-co-1,12 dodecane diol dodecanedioate) PDDD random copolyester, using 1,4 dithiane 2,5 diol as versatile monomer, with the aim of establishing biological properties which are very useful to design a material for a biomedical application.
Experimental Section
Materials and Methods
Sigma Aldrich samples of 1,4 dithiane 2,5 diol, 1,12 dodecane dioic acid and 1,12 dodecane diol were purchased. Titanium Tetra isopropoxide were purchased from Lancaster and used as catalyst. The chemicals and solvents of AR Grade were used as such. Ubbelohde Viscometer was used to determine inherent viscosity of copolyester. FT-IR Spectra was executed on Perkin Elmer 883 Spectrophotometer. 1H-NMR spectra of the copolyester using CDCl3 as a solvent was recorded on a Bruker 400 MHz Spectrometer. DSC Q200 V23.10 Build 79 Differential Scanning Calorimeter and Bruker B8 wide angle XRD with Cu/30kv/15mA were used to record the DSC thermogram and XRD diffractogram. The in Vitro cytotoxicity of the synthesised copolyester against vero cell line and lung cancer cell line was carried out by MTT Assay Method (Mosmann, 1983).23 The antioxidant and antimicrobial activity of the copolyester was determined by DPPH Scavenging Assay (Dot blot assay24 and Spectrophotometer) and Well diffusion method25 respectively.
Synthesis of Copolyester
1,4 dithiane 2,5 diol (0.01 mole), 1,12 dodecane diol (0.01mole) and 1,12 dodecane dioic acid (0.02 mole) were taken in a three necked round bottom flask and kept in an oil bath. N2 gas was passed through the left inlet and the guard tube filled with calcium chloride is connected the middle inlet and the right inlet was closed with a stopper. The mixture was heated upto its melting with continuous stirring and then about 0.8 mL of titanium tetra isopropoxide was added and the temperature was maintained at 130°C for one hour. Then the temperature was increased to 140°C and maintained for 2 hours. The crude copolyester produced in the reaction flask was dissolved in chloroform and then poured in ice cold methanol with stirring to reprecipitate the copolyester, which is then filtered and dried. The copolyester PDDD was synthesized as per the Scheme 1.
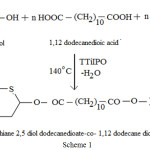 |
Scheme 1 |
Results and Discussion
Solubility and Viscosity Studies
The synthesized copolyester was dissolved in various solvents to determine its solubility. The copolyester was soluble in Chloroform, DMF and THF, while partially soluble in acetone and carbon tetrachloride. The inherent viscosity of the polymer PDDD was confirmed by determining the flow time of the polymer at the concentration of 1mg/mL and the chloroform as solvent using Ubbelohde Viscometer. The inherent viscosity of the copolyester was determined as 0.984 dL/g.
FT-IR Spectral Studies
The FT-IR spectrum of the copolyester PDDD is shown in Figure 1. A strong absorption band at around 1728 cm-1 corresponds to the carbonyl stretching vibration of ester group in copolyester. The absorption bands at 2936cm-1 and 1130cm-1 are assigned to aliphatic C-H stretching of the diacids/diols and C-O stretching of ester group. The absorption bands at 780 cm-1, 659 cm-1 and 1420 cm-1 were attributed to C-H bending of 1, 4 disubsituted dithane, C-S stretching of dithiane moiety and aliphatic C-C stretching respectively. It is evident from the IR spectra that the sulphur containing monomeric units and the ester group are present in the copolyester.
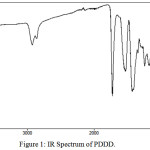 |
Figure 1: IR Spectrum of PDDD. |
1H NMR Spectral Studies
The 1H NMR spectra of the copolyester PDDD was shown in Fig 2. The 1H NMR spectrum exhibits characteristic peaks at 1.25-1.38 ppm and 1.57-1.63 ppm corresponding to the methylene protons of diol and dioic acid respectively. The peak at 2.34- 2.37 ppm was due to the –CH2-CO- protons while the peak at 2.82- 3.37 was attributed –CH2-S protons of 1, 4 dithiane 2,5 diol, while the peaks at 3.7-4.02 ppm and 4.27 ppm was attributed to–CH2-O protons and > CH-O protons of 1,4-dithiane 2,5-diol respectively. The presence of the versatile monomer moiety in the copolyester are confirmed in accordance with the individual 1H NMR spectrum of 1,4-dithiane 2,5- diol.
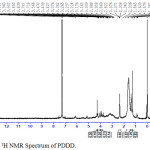 |
Figure 2: 1H NMR Spectrum of PDDD. |
DSC Studies
The Thermal properties of the copolyester was determined by the Differential Scanning Calorimetry. The glass transition and the melting point temperature generally decreases with increase in methylene groups and it was confirmed in the synthesised copolyester with Tg as -72°C and its melting point at 114.1°C, which was shown in the Fig. 3. The lower Tg value indicates that the synthesised copolyester have flexible polymeric chains26 and can be used in drug delivery applications.27
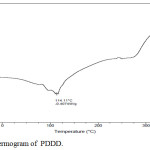 |
Figure 3: DSC Thermogram of PDDD. |
Wide Angle X- Ray Diffraction Studies
Wide angle X-ray diffraction analysis was an effective tool to investigate the degree of crystallinity of polymer. The copolyester exhibits a amorphous halo at 2θ range of about 15°C to 23°C and it is evident from X-ray diffractogram as shown in Fig. 4. The X- ray diffractogram of copolyester PDDD exhibits the amorphous nature of copolyester.28
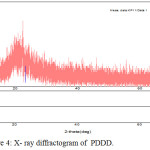 |
Figure 4: X-ray diffractogram of PDDD. |
Antioxidant Activity
The antioxidant activity of copolyester PDDD was determined by DPPH scavenging assay on TLC by dot blot method as shown in Fig. 5 and by Spectrophotometer. DPPH scavenging assay on TLC by dot blot method showed a color change from purple to yellow, as the antioxidant compound can donate hydrogen atom to the DPPH radical and turns purple color of DPPH radical into yellow color of DPPH-H radical. The amount of scavenging potential from purple to yellow indicates the antioxidant activity of copolyester. The DPPH Scavenging Assay by Spectrophotometer for synthesized Copolyester PDDD showed a decrease in absorbance from DPPH radical to DPPH-H form. The maximum percentage of inhibition activity was determined for various concentrations (1000- 15.62µg/mL) of copolyester and was given in Table 1. The maximum percentage of inhibition activity of copolyester was 81.60% (Table 1) at the concentration of 1000 µg/mL, whereas standard showed maximum percentage of Inhibition activity 75.85% (quercetin) at the concentration of 10 µg/mL. The antioxidant activity of PDDD might be attributed to their hydrogen donating ability to DPPH free radicals. The lower the IC50 value and higher the % of inhibition, the greater is its antioxidant activity.29 The IC50 value (129.21) of the copolyester indicates that it has excellent antioxidant activity. Antioxidant compounds are the one which protects human, animal and plant cells from the damaging effects of free radicals reactive oxygen species (ROS). Oxidative stress occurs when there was an imbalance between antioxidants and free radical, which may lead to cellular damage (Kukic et al., 2006). The antioxidant compound also helps to reduce the occurrence of stroke, heart failure, diabetes and cancer.
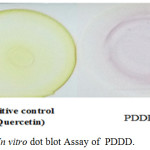 |
Figure 5: In vitro dot blot Assay of PDDD. |
Table 1: In vitro DPPH Activities of PDDD.
|
Concentration of copolyester (µg/ml) |
% Inhibition |
|
1000 |
81.60±5.71 |
|
500 |
62.31±4.36 |
|
250 |
53.71±3.76 |
|
125 |
48.37±3.39 |
|
31.2 |
38.58±2.70 |
|
15.6 |
35.61±2.49 |
|
Cell Control |
20.62±1.44 |
|
IC 50 Value (µg/ml) |
129.21 |
Antimicrobial Activity
The In vitro antimicrobial activity of the synthesised copolyester PDDD was determined by well diffusion method as described by Perez et al. (1990) using Mueller Hinton Agar (MHA) medium for four bacterial strains Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus and Bacillus subtilis and the images are shown in Fig. 6. The higher the zone of inhibition the greater is the antimicrobial activity.30,31 The copolyester showed various range of zone of inhibition at different concentrations (250,500, 1000 µg) against human pathogens. The synthesized copolyester has exhibited inhibition zones ranging from 10 to 16 mm, which shows that it has excellant antimicrobial activity. The copolyester has showed more antimicrobial activity towards Escherichia coli at all concentrations when compared to other human pathogens. The rate of inhibition was found to be higher for Bacillus subtilis at 1000 µg/mL as compared to all bacterial strains.
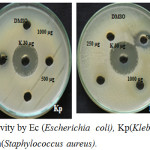 |
Figure 6: Antimicrobial activity by Ec (Escherichia coli), Kp(Klebsiella pneumoniae), Bs (Bacillus subtilis) and Sa(Staphylococcus aureus). |
Table 2: Antimicrobial activity of PDDD by well diffusion method.
| Human Pathogen |
Concentration (µg/ml) |
Zone of inhibition (mm) |
% of inhibition |
Kannamycin (30 µg) Zone of inhibition (% of inhibition) |
| Escherichia coli |
1000 |
14±0.98 |
15.55±1.08 |
|
|
500 |
12±0.84 |
13.33±0.9 |
26.33±1.52 (29.25±1.38) |
|
|
250 |
10±0.7 |
11.11±0.7 |
||
| Klebsiellapneumonia |
1000 |
12±0.84 |
13.33±0.93 |
|
|
500 |
– |
– |
30.67±1.52 (34.07±1.38) |
|
|
250 |
– |
– |
||
| Staphylococcusaureus |
1000 |
11±0.77 |
12.22±0.85 |
|
|
500 |
– |
– |
26.00±1.00 (29.25±0.52) |
|
|
250 |
– |
– |
||
| Bacillus subtilis |
1000 |
16±1.12 |
17.78±1.24 |
|
|
500 |
12±0.84 |
13.33±0.9 |
27.00±1.00 (30.00±0.90) |
|
|
250 |
– |
– |
Anticancer Activity
The anticancer activity of the copolyester was investigated by MTT assay method, in which the % cell viability for different concentration of the copolyester and IC50 values for Vero and A549 cell line was determined as shown in Table 3 & 4. In Fig. 7 and Fig. 8, a graphical representation of copolyester effect on Vero and A549 cell line by % cell viability was shown. The affected Vero and A549 cell line at different concentration was shown in Fig. 9 & 10. The PDDD copolyester exhibited IC50 value for Vero cell line and A549 cell line at 972.95 μg/ml and 119.32 μg/ml respectively. The lower value of IC50 for A549 (lung cancer cell line) than Vero (Normal cell line) indicates that the copolyester have excellent anticancer activity on cancer cells.32
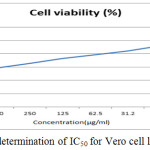 |
Figure 7: Graphical determination of IC50 for Vero cell line. |
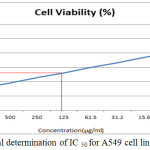 |
Figure 8: Graphical determination of IC 50 for A549 cell line.Click here to view figure |
Table 3: Anticancer activity of PDDD on Vero cell line.
|
Concentration (µg/mL) |
Dilutions |
Absorption(OD) |
% Cell Viability |
|
1000 |
Neat |
0.240 |
51.39 |
|
500 |
1:1 |
0.274 |
58.67 |
|
250 |
1:2 |
0.305 |
65.31 |
|
125 |
1:4 |
0.339 |
72.59 |
|
62.5 |
1:8 |
0.366 |
78.37 |
|
31.25 |
1:16 |
0.394 |
84.36 |
|
15.62 |
1:32 |
0.429 |
91.86 |
|
7.8 |
1:64 |
0.455 |
97.43 |
|
Cell Control |
– |
0.467 |
100 |
|
IC 50(µg/mL) |
– |
972.95 |
Table 4: Anticancer activity of PDDD on A549 cell line.
|
Concentration (µg/mL) |
Dilutions |
Absorption(OD) |
% Cell Viability |
|
1000 |
Neat |
0.257 |
31.57 |
|
500 |
1:1 |
0.312 |
38.18 |
|
250 |
1:2 |
0.374 |
45.77 |
|
125 |
1:4 |
0.428 |
52.38 |
|
62.5 |
1:8 |
0.487 |
59.60 |
|
31.25 |
1:16 |
0.544 |
66.58 |
|
15.62 |
1:32 |
0.603 |
73.80 |
|
7.8 |
1:64 |
0.659 |
80.66 |
|
Cell Control |
– |
0.817 |
100 |
|
IC 50(µg/mL) |
– |
– |
119.32 |
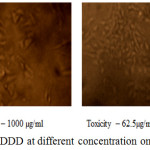 |
Figure 9: Anticancer activity of PDDD at different concentration on Vero cell line. |
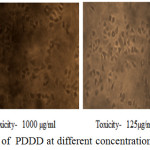 |
Figure 10: Anticancer activity of PDDD at different concentration on A549 cell line. |
Conclusion
The biodegradable aliphatic random copolyester PDDD was synthesized by direct melt polycondensation method and the repeating units in structure of copolyester was confirmed by FT-IR and NMR spectroscopy. The inherent viscosity of the PDDD determined by using ubbelohde viscometer showed that it has high degree of polymerization. The lower Tg value of the copolyester shows that it has flexible polymeric chains and hence can be used in drug delivery applications. The X ray diffraction studies revealed that copolyester PDDD is amorphous in nature. The synthesized copolyester PDDD using versatile monomer 1,4 dithiane 2,5 diol have exhibited antioxidant, antimicrobial and anticancer activity, which shows that it can used in various biomedical application.
Acknowledgements
The authors thank Central Leather Research Institute, Chennai and Vellore Institute of Technology, Vellore for providing Instrumentation facilities.
Conflict of Interest
There is no conflict of interest.
References
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Polymer Degradation and Stability. 2006, 91(2), 367–376.
- Lu, T. S.; Sun, Y. M.; Wang, C. S. J. Polym. Sci. A. 1995, 33(16), 2841–2850.
- Byrom, D. Trends in Biotechnol. 1987, 5(9), 246–250.
- Raquez, J. M.; Nabar, Y.; Narayan, R.; Dubois, P. Macromol. Mater. Eng. 2008, 293(4), 310–320.
- Ulery, B. D.; Nair, L. S.; Laurencin, C. T. J. Polym. Sci. Part B. 2011, 49(2), 832–864.
- Seyednejad, H.; Ghassemi, A. H.; Van Nostrum, C. F.; Vermonden, T.; Hennink, W. E. J. Control. Release. 2011, 152(1), 168–176.
- Vert, M. Biomacromolecules. 2005, 6(2), 538–546.
- Kausar, A.; Zulfiqar, S.; Ilyas Sarwar, M. Polymer Reviews. 2014, 54(2), 185-267.
- Steinbuchel, A.; Lutke-Eversloh, T.; Ewering, C. US Patent 6495152 B2, 2002, Dec 17.
- Jha, G. S.; Seshadri, G.; Mohan, A.; Khandal, R. k. e-Polymers. 2008, 8(1), 2197-4586.
- Tanaka, M.; Kuma, S.; Kobayashi, S.; Kanemura, Y. US6770735B2, 2004, Aug 3.
- Kim, Y. S.; Kim, J. C.; Seo, H. H.; Choi, G. J.; Park, A. R. WO2008120955A, 2008, Oct 9.
- Hirano, H.; Kadota, J.; Agari, Y.; Harada, T.; Tanaka, M.; Hasegawa, K. Polymer Engineering and Science. 2007, 47(3), 262-269.
- Seiichi, K.; Hiroyuki, M. US7132501B2, 2006, Nov 7.
- Yao, H.; Lee, S. D. US20070020557A1, 2007, Jan 25.
- Edward, D.; David L. M.; Michael, E. B. US6149678A, 2000, Nov 21.
- Blakey, I.; Conley, I.; George, G. A.; Hill, D. J. T.; Liu, H.; Rasoul, F.; Whittaker, A. K. Proceedings SPIE, Advances in Resist Technology and Proceedings XXIII. 2006, 6153.
- Hans, J. L.; Jens, K.; Reinhard, H.; Christian, W.; Dorota, G.F. US20110281965A1, 2011, Nov 17.
- Kayser, K. J.; Cleveland, L.; Park, H. S.; Kwak, J. K.; Kolhatkar, A.; Kilbane II, J. J. Appl Microbiol Biotechnol. 2002, 59(6), 737-745.
- Hwang, K. J.; Lee, T. S.; Kim, K. W.; Kim, B. T.; Lee, C. M.; Park, E. Y. Arch Pharm Res. 2001, 24(4), 270-275.
- Yijun, H.; Siglinde, W.; Michal, B.; Lidio, M.; Carlos, C.; Tad, A. H.; Alexander, D. Chem. Biol. Drug Des. 2010, 76(2), 116-129.
- Erik, D.C. Pure Appl. Chem. 2001, 73(1), 55-66.
- Mosmann, T. J. Immunol. Methods. 1983, 65(1-2), 55-63.
- Blois, M.S. Nature. 1958, 181, 1199-1200.
- Perez, C.; Pauli, M.; Bazerque, P.S. Acta. Biol. Med. Exp. 1990, 15, 113-115.
- Rizzarelli, P.; Puglisi, C.; Montaudo, G. Polymer Degradation and Stability. 2004, 85(2), 855-863.
- Karavelidis, V.; Giliopoulos, D.; Karavas, E.; Bikiaris, D. European Journal of Pharmaceutical Sciences. 2010, 41(5), 636-643.
- Sudhakar, K.; Nanthini, R.; Asian Journal of Chemistry, 2018, 30(1), 191-194.
- Sinaga, S. M.; Sudarmi, S.; Iksen, I.; Kevin, K.; Sari, M. P. Rasayan J. Chem. 2018, 11(4), 1604 – 1608.
- Hossein, J.; Haron, M. D. J.; Halem, M.; Ismail, M. H. S.; Roshanak, R. M.; Leili, A. H.; Yadollah, A.; Majid, R.; Nazanin, V. Digest Journal of Nanomaterials and Biostructures. 2013, 8(3), 1263 – 1270.
- Parekh, J.; Chanda, S. Braz. J. Microbiol. 2007, 38(2), 204-207.
- Ariharan, V. N.; Prasad, P. N. Rasayan. J. Chem. 2014, 7(3), 260-263.

This work is licensed under a Creative Commons Attribution 4.0 International License.









