The Role of Micropores and Amine Groups in Preferential CO2 Adsorptivity of Porous Zn-Coordination Polymers Comprising Mixed Ligands of Triazole and Amino Triazole
Moondra Zubir1 , Hafni Indriati Nasution1 and Teguh Febri Sudarma2
, Hafni Indriati Nasution1 and Teguh Febri Sudarma2
1Department of Chemistry, Faculty of Mathematics and Natural Science, State University of Medan, Jl. Willem Iskandar, Pasar V, Medan Estate, Medan, North Sumatera 20221, Indonesia.
2Department of Physics, Faculty of Mathematics and Natural Science, State University of Medan, Jl. Willem Iskandar,Pasar V, Medan Estate, Medan, North Sumatera 20221, Indonesia.
Corresponding Author E-mail: moondrazubir@unimed.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350158
Article Received on : 24-11-2018
Article Accepted on : 11-01-2019
Article Published : 25 Jan 2019
Porous coordination polymers (PCPs) of Zn2+, oxalic acid and mixture ratio of 3-amino, 1,2,4-triazole (Ataz) and 1,2,4-triazole (Taz) were synthesized to capture of carbon dioxide. A series of molar fraction Taz / (Taz + ATaz) prepared as 0.1;0.3;0.4;0.5;0.6;0.7 and 0.9 and also only ATaz and Taz ligand as 0 and 1 molar fraction respectively. Mixture of Taz and ATaz crystal induce new structure of 0.4, 0.5 and 0.6 molar fraction. Nitrogen adsorption of 50 % mixture each ligand contribute the highest surface area as function of optimum condition to integrate pore space and amine group presence with adsorbed as 290 mgg-1. This phenomena also shown through CO2 adsorption amount as 135 mgg-1, instead of 0.4, 0.6 and 0.7 molar fraction observed higher amount of CO2 compared with only Taz ligand (X=1), indicate the integrated effects both of the ligand could generated the capture of higher amount of CO2.
KEYWORDS:Adsorption; Amino Triazole; Carbon Dioxide; Mixed Ligands; Triazole
Download this article as:| Copy the following to cite this article: Zubir M, Nasution H. I, Sudarma T. F. The Role of Micropores and Amine Groups in Preferential CO2 Adsorptivity of Porous Zn-Coordination Polymers Comprising Mixed Ligands of Triazole and Amino Triazole. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Zubir M, Nasution H. I, Sudarma T. F. The Role of Micropores and Amine Groups in Preferential CO2 Adsorptivity of Porous Zn-Coordination Polymers Comprising Mixed Ligands of Triazole and Amino Triazole. Orient J Chem 2019;35(1). Available from: https://bit.ly/2Dx3uVQ |
Introduction
Porous coordination polymers (PCPs), are novel materials constructed by coordinate bonds between multi-dentate ligands and metal atoms became new attractive porous materials as highly selective gas adsorption capability.1-6 Some of results have indicated that porous coordination polymers (PCPs) might become promising adsorbents for CO2 separation.7-12 Considerable effort has been reported on the synthesis of PCP materials in the last several years.They are synthesized mainly by hydrothermal or solvothermal methods and the interesting synthesis of this material while choice of metal centers and design and synthesis of ligands. Different combinations of metal centers and ligands based on rational design ideas will generate PCP materials with various structures and preferential pore properties. Besides large surface areas and pore volumes, many PCP materials are well known to have specific interaction of CO2 with functionalized aromatic molecules has shown the effectiveness of introducing specific polar substituent groups, e.g. –OH, -COOH, -CH3 or NH2, on the aromatic ligand in increasing the CO2-ligand affinity.13,14
However, only a limited number of reports on frameworks having more than two kinds of ligands. Some of the reports about mixing ligand system was synthesized as Zn4O(bdc)x(abdc)3-x, in which the terepthalic linkers are partially substituted by 2-aminobenzene-1,4-dicarboxylate (abdc).Otherwise, the potential of the mixed-ligand approach in the framework of Z4O(L)3 (L = terephthalic acid derivatives) was extended by use of a high-throughput technique.15 In the frameworks of the structure, various terephthalic acid derivatives can be incorporated to create a porous structure, and one of them has eight terephthalic acid derivatives in the crystal structure. They suggest that the matching of the interval of ligands and comparable strengths of coordination bonds are the key to integrating the different ligands in the structure.
Comprising of Zn2+, oxalic acid and 3-amino, 1,2,4-triazole was synthesized as flexible amine functionalized PCP.11 These three dimensional frameworks built from the pillaring of Zn-aminotriazolate layers by the oxalate groups. The oxalate ligands bind to the zinc in a bidentate mode through two oxygen atom from different carboxylate groups. The aminotriazolate ligand bind to trigonal bipyramidal zinc centres through the three N-atoms of the ring, while the amino groups remain. This frameworks does not appear to be porous shown from did not show any appreciable uptake of N2, Ar or H2 under comparable condition. It possible because of presence of free amine groups inside the pore should blocked to penetrate of guest molecules. Interestingly, presence of amine groups create significant amount of carbon dioxide (CO2) to capture in the frameworks. The potential of amine groups to interact with CO2 molecules is by now reasonably well understood. In previously report, we have explored the new design of PCPs as Zn2(C2O4)(C2N3H2)2.(H2O)2.5 or Zn-oxac-Taz 12,17 with triazole ligands with no amine groups presence which promoted significant N2 amount adsorption isotherms and also available space to accept carbon dioxide molecules. If compare with others PCPs as Zn2(C2O4)(C2N4H3)2.(H2O)0.511 or Zn-oxac-ATaz which have amine groups in ligands structure, no space observed for nitrogen adsorption isotherms even could capture CO2 molecules due to their amine groups presence inside the pore interacted with CO2 molecules.
In this study, we observed how PCPs were synthesized with two different properties of ligands could produce preferential framework of pore space and functional group combination effect in enhancing the CO2 absorptivity. Herein, PCPs were synthesized to confirm the effects of amine groups interaction with CO2 molecules by increasing pore space from 2 ligands of 1,2,4-Triazole (Taz) with no amine groups but create larger space were combined with 3-Amino, 1,2,4-Triazole (ataz) ligands with amine groups presence inside the pore. Mixture comparison of nTaz/n(Taz+ATaz) as 0.1, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.9 and to compare with ATaz and Taz ligand contain only, molar fraction mention as 0 for only ATaz ligand and 1 for only Taz ligand. Interesting phenomena observed by N2 and CO2 adsorption isotherms to give new flexibility pore properties of Porous Coordination Polymers (PCPs).
Materials and Methods
Mixed ligand PCPs of 0.1 molar fraction was synthesized by 0.4 g of Zn5(CO3)2(OH)6 (Alfa Aesar, Co. Ltd.; 75% purity), 0.4 g of H2C2O4 (Wako Pure Chemical Industries (WPCI), Ltd.), 0.14 g of 1,2,4-triazole (WPCI, Ltd.) and 1.44 g 3-amino, 1,2,4-triazole (WPCI. Ltd) were added to a mixed solution of 12 mL of methanol (WPCI, Ltd.; 99.5%) and 2 mL of distilled water. The solution containing white precipitates was transferred to PFA cell which was set in autoclave vessel and heated at 453 K for 12 hours. Then, cooling the system to room temperature, crystals prepared were filtered and washed with a mixture of methanol and water, followed by drying it at room temperature overnight.
The same procedure were used for synthesis of mixed ligands PCPs with molar fraction 0.3, 0.4, 0.5, 0.7 and 0.9 by variation change the weight of 1,2,4-triazole (Taz) and 3-amino, 1,2,4-triazole (ATaz).
Crystals prepared were characterized by a Rigaku X-ray diffractometer (XRD) Multiflex with Cu-Kα at 40 kV and 20 mA and their morphologies were observed by using a JEOL scanning electron microscopy (SEM) JSM-7600 F. Thermogravimetric and differential thermal analyses (TG/DTA) were carried out using a Rigaku Thermo Plus TG8120. Samples enclosed in Al-pans were heated at 10 K/min from 298 to 723 K in air using Al2O3 as a reference.
N2 and CO2 adsorption were measured at 77 and 303 K, respectively, by a custom-made volumetric adsorption system after each sample of ca. 100 mg was pretreated at 333 K and 1 mPa for 1 h and then 383 K for 12 h.
Results and Discussion
Gradually increase of Taz ligand presence at 0.1 and 0.3 molar fraction shows only a slight change of XRD pattern, indicate no significant crystal structure changed or similar with only ATaz ligand (X=0). The same case for 0.7 and 0.9 molar fraction, XRD patters almost similar with only Taz ligand presence (X=1). The mixture of Taz and ATaz crystal induced new structure of 0.4, 0.5 and 0.6 molar fraction as can be seen in Fig. 1. By using EXPO 2013,18 crystal system measured and only for X=0.1 molar fraction sample (only Taz ligand) formed as monoclinic crystal, then X=0 (ATaz), 0.1, and 0.3 as orthorombic crystal system. Instead for 0.4, 0.5 and 0.6 molar fraction crystal formed as triclinic crystal system, similar to 0.7 and 0.9 molar fraction PCPs.
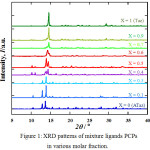 |
Figure 1: XRD patterns of mixture ligands PCPs in various molar fraction. |
To confirm this crystal structure changes, SEM image also was observed in the same magnification and the specific phenomena shown in morphology properties (Fig. 2). Similar to XRD patterns, almost identical particle formed for 0, 0.1 and 0.3 molar fraction sample. Molar fraction sample of 0.9 and 1 also observed a similar morphology and a little bit different for 0.7 molar fraction sample, indicate the changed of peak intensity in XRD patterns make a slightly different of morphology. The completely different morphology observed in 0.4, 0.5, and 0.6 molar fraction samples. By using almost same comparison both of Taz and ATaz ligands induce the new structure and morphology to create integrating effects of pore space and amine group presences in the frameworks.
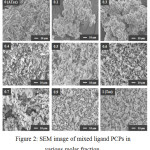 |
Figure 2: SEM image of mixed ligand PCPs in various molar fraction. |
Figure 3 shows that pretreatment for 12 h before N2 adsorption of PCPs with molar fraction 0.4, 0.5, 0.6, 0.7, 0.9 and 1 (Taz only) brought about significantly increased the adsorption amount from low pressure, whose adsorption isotherms are classified as type I. Presence more Taz ligands induce more space in the PCPs frameworks. On the other hand, PCPs with ATaz Ligands (X=0) and PCPs with molar fraction 0.1, 0.2 and 0.3 shows a pretreatment for 12h brought about little change in the adsorption amount of N2, but gradually increased at high pressure indicate presence of amine group block N2 to penetrate inside the frameworks.
The new structure of 50% mixture ATaz and Taz ligand also bring out the high surface area and pore volume by nitrogen adsorption isotherms at 77K as shown in Fig. 3, even though Zn-oxac-Taz (X=1) which no amine group has highest surface area, indicate more space formed with use ligands without amine group. In the other hand, nitrogen adsorption amount which almost no adsorption of nitrogen in 0.1 and 0.3 as similar with only ATaz ligand (0). Nitrogen could penetrate to the frameworks after 40 % Taz ligand (X=0.4) exist, it suggest to create the pore space which enough for nitrogen molecules. And the surface area gradually decrease by presence of 10%, 30% and 40% ATaz ligand in framework for 0.9, 0.7 and 0.6 molar fraction respectively if compared to PCP with only Taz ligand (X=1). It confirm to presences of amine group inside the frameworks reduce the space of pores.
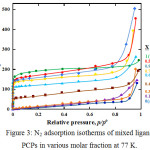 |
Figure 3: N2 adsorption isotherms of mixed ligand PCPs in various molar fraction at 77 K. |
Fig. 4 shows the langmuir adsorption isotherms type through the integration effects of pore size and amine groups presences inside the frameworks more inexplicable by carbon dioxide adsorption isotherms.
Even no nitrogen amount adsorbed in 0, 0.1 and 0.3 molar fraction crystal, significant amount of CO2 can adsorbed which contribute from the CO2 and –NH2 interaction. And exist of 30 % Taz ligand could make a little space and made CO2 molecules can access to the pores, indicate by slightly increase of CO2 adsorption amount. More interestingly, even 0.4, 0.6, 0.7 and 0.9 molar fraction crystal have lower adsorption amount of nitrogen if compared to PCP with only Taz ligand presence (X=1), indicate lower surface area or pore volume but could be adsorb higher CO2 molecules. It confirm CO2 and –NH2 interaction have significant effects to increase CO2 capacity inside the frameworks.
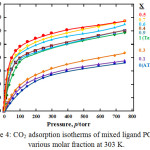 |
Figure 4: CO2 adsorption isotherms of mixed ligand PCPs in various molar fraction at 303 K. |
Micropore volume, BET Surface area and Langmuir Surface area were summarized in Table 1 and Fig. 5. It clarified the effects of Taz ligand function act as pore space created which describe by nitrogen adsorption amount and amine group presence from ATaz ligand has big contribution to enhance the carbon dioxide adsorption amount. By increasing the surface area or provide bigger space inside the frameworks more effectives to enhance CO2 amount indicated by higher amount of CO2 adsorption over 0.4 molar fraction. Even though, the presence of an equal number of Taz and ATaz ligand become optimum condition not only to create the biggest surface area and pore volume but also the highest CO2 molecules adsorb inside the frameworks.
Table 1: Summary of gas adsorption data.
| Molar Fraction | CO2 adsorption | N2 adsorption | |||||
| adsorbed amount at 100 torr (mg g-1) | adsorbed amount at 700 torr (mg g-1) | SALang (m2 g-1) | adsorbed amount at 0.1 P/Po | Micropore Volume * (cm3 g-1) | Pore Size * D (nm) | SABET (m2 g-1) | |
| 0 (ATaz) | 31.3 | 72.8 | 214 | 6.6 | – | – | – |
| 0.1 | 33.3 | 77.8 | 224 | 8.5 | – | – | – |
| 0.3 | 39.5 | 87.2 | 257 | 10.9 | – | – | – |
| 0.4 | 78.8 | 120.3 | 354 | 65.6 | 0.09 | 1.08 | 185 |
| 0.5 | 97.1 | 135 | 398 | 290.1 | 0.37 | 0.77 | 544 |
| 0.6 | 86.9 | 130.3 | 384 | 120.3 | 0.15 | 0.72 | 311 |
| 0.7 | 90.9 | 134.8 | 397 | 145.0 | 0.18 | 0.75 | 380 |
| 0.9 | 78.7 | 121.7 | 358 | 155.0 | 0.19 | 0.69 | 408 |
| 1 (Taz) | 73.4 | 117.4 | 346 | 185.2 | 0.23 | 0.84 | 494 |
*micropore volume estimated by DR plot analysis and pore size by t-plot analysis of N2 adsorption isotherms at 77K.
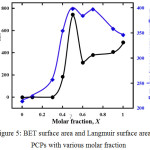 |
Figure 5: BET surface area and Langmuir surface area all PCPs with various molar fraction. |
At a ratio of 50% ATaz and Taz ligand (X = 0.5) induce the highest BET surface area is 544 m2g-1 due to the presence of amine ligands in the cavity making a larger pore volume which caused by the interaction of the arrangement presence of amine groups in the pore supported by sufficient size of space from Taz ligand to make a larger cavity. BET surface area of PCP made from ligand comparisons X = 0 (Taz) to X=0.4 is smaller than X = 0.6 to X=1 (ATaz), which indicate the presence of more amine groups were blocked nitrogen gas to penetrate inside the pores. Decrease of the presence of amine groups increasing the BET surface area in PCPs with molar fraction, X = 0.6 – 1 (Ataz) although lower than the PCP made with the same ratio or X = 0.5. It confirmed the pore size formed by the presence of 50% ligand ATaz and Taz due to this flexible structure of these PCPs series.11,12,17
It is also observed in the Langmuir surface area, CO2 was absorbed at larger pore sizes with the presence of amine groups in the pore. However, despite the pore volume of 0.37 cm3g-1 formed in PCP with a ligand ratio of X = 0.5, the largest pore size was observed in PCP with a ligand ratio of X = 0.4 as 1.08 nm. (Fig. 6). Almost no pore formed in PCPs were synthesized from a mixture of ligands with a molar fraction of 0(Ataz) to X=0.3 because the presence of amine groups still blocked the pores from the guest molecule interpenetrate. PCPs were synthesized of Taz/ATaz 0.4 molar fraction, induce to maximum pore size formation, then PCP was formed by a molar fraction of 0.5 has a larger pore volume. It suggest how flexible the PCP structure is easily to modified by comparison of ligand composition.
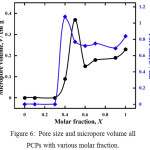 |
Figure 6: Pore size and micropore volume all PCPs with various molar fraction. |
Conclusion
A series of new PCP design from mixture ATaz and Taz ligand can explain how strong the amine groups interaction with carbon dioxide through bigger pore space needed to allow carbon dioxide molecule through inside the pores. By increasing the surface area from Taz ligand and presence of amine groups up to 60% from ATaz ligand more effective to increase to capture the CO2 molecules. PCPs prepared in 50 % contain both of ligand shown the best composition to get the highest not only surface area, but also the carbon dioxide capacity. It become the new strategy to prepare PCP which have preferential properties for CO2 adsorption.
References
- Kitagawa S.; Kitaura R.; Noro R. Angew. Chem. Int.Ed., 2014, 43, 2334-2375.
- Shimomura S.; Horike S.; Matsuda R.; Kitagawa S. Journal of American Chemical Society, 2007, 129, 10990-10991.
- Li H.; Eddaoudi M.; Groy T.L.; Yaghi O.M. Journal of American Chemical Society, 1998, 120.
- Li W.; Jia H.P.; Ju Z.; Zhang J.A. Crystal Growth & Design, 2006, 6,(9); 2136-2140.
- Garcia-Ricard O.J.; Morales P.M.; Martinez J.C.S.; Curet-Arana M.C.J.; Hogan A.; Hernandez-Maldonado A.J. Microporous and Mesoporous Materials. 2013, 177, 54-58.
- Noro S.; Kitagawa S.; Akutagawa T.; Nakamura T. Prog. Polym. Sci. 2009, 34, 240-279.
- Millward A.R.; Yaghi O.M. Journal of American Chemical Society. 2005, 127, 17998-17999.
- Li H.L; Eddaoudi M.; O’Keeffe M.; Yaghi O.M. Nature. 1999, 402, 276–279.
- Yang Q.Y.; Xue C.Y.; Zhong, C.L.; Chen J.F. AICHE J., 2007, 53, 2832–2840.
- Arstad B.; Fjellvag H.; Kongshaug K.O.; Swang O.; Blom R. Adsorption. 2008, 14, 755-762.
- Vaidhyanathan R.; Iremonger S.S.; Dawson K.W.; Shimizu G.K.H. Chem. Commun. 2009, 5230-5232.
- Zubir M.; Hamasaki A.; Ohta A.; Ohki H.; Ozeki S. Langmuir, 2017, 33, 680−684.
- Eddaoudi M.; Kim J.; Rosi N.L.; Vodak D.; Wachter J.; O’Keeffe M.; Yaghi O.M. Science. 2002, 295, 469–472.
- Rosi N.L.; Eckert J.; Eddaoudi M.; Vodak D.T.; Kim J.; O’Keeffe M.; Yaghi O.M. Science. 2003, 300, 1127–1129.
- Kleist W.; Jutz F.; Maciejewski M.; Baiker A. Eur. J. Inor.Chem. 2009, 24, 3552-3561.
- Deng H.X.; Doonan C.J.; Furukawa H.; Ferreira R.B.; Towne J.; Knobler C.B.; Wang B.; Yaghi O.M. Science. 2010, 327, 846-850.
- Zubir M.; Hamasaki A.; Iiyama T.; Ohta A.; Ohki H.; Ozeki S. Chemistry Letters., 2016, 45, 362-364.
- Altomare A.; Cuocci C.; Giacovazzo C.; Moliterni A.; Rizzi R.; Corriero N.; Falcicchio. A. J. Appl. Cryst., 2013, 46, 1231-1235.

This work is licensed under a Creative Commons Attribution 4.0 International License.









