Synthesis, Characterization and Thermal Stability Study of New Heterocyclic Compounds Containing 1,2,3-Triazole and 1,3,4-Thiadiazole Rings
Riyadh J. Nahi and Noor H. Imran
Department of Chemistry, College of Science, Al Muthanna University, Iraq.
Corresponding Author E-mail: riyadhnahi@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350128
Article Received on : 28-11-2018
Article Accepted on : 30-01-2019
Article Published : 26 Feb 2019
In the current study, a new series of 1,2,3-triazole derivatives was synthesized via copper (I) catalyzed azide-alkyne cycloaddition reaction using a series of synthesised p-substituted phenyl azides and propiolic acid. In addition, a new series of heterocyclic compounds containing 1,2,3-triazole and 1,3,4-thiadiazole ring combined in one molecule was synthesised via a condensation reaction of the synthesised 1,2,3-triazole derivatives and thiosemicarbazide in the presence of POCl3. Furthermore, the thermal behavior of these newly synthesised compounds was studied by TGA and DTG techniques.
KEYWORDS:Click Reaction; CuAAC; Thermal Stability; 1,2,3-Triazole; 1,3,4-Thiadiazole
Download this article as:| Copy the following to cite this article: Nahi R. J, Imran N. H. Synthesis, Characterization and Thermal Stability Study of New Heterocyclic Compounds Containing 1,2,3-Triazole and 1,3,4-Thiadiazole Rings. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Nahi R. J, Imran N. H. Synthesis, Characterization and Thermal Stability Study of New Heterocyclic Compounds Containing 1,2,3-Triazole and 1,3,4-Thiadiazole Rings. Orient J Chem 2019;35(1). Available from: https://bit.ly/2T1h4dm |
Introduction
Heterocyclic compounds are a major class of organic compounds that are identified by the fact that their structures composed of at least one ring system contains at least one non-carbon atom such as oxygen (O), nitrogen (N) and sulpher (S) as heteroatoms.1–3 Azoles ring system containing heterocyclic compounds have a special importance due to a wide range of different applications.4,5 Azole is a five-membered ring containing nitrogen atom and at least one other non-carbon atom of either nitrogen, sulphur, or oxygen.6-8 Among many different azole structures, 1,4-disubstituted-1,2,3-triazole and 2-amino-1,3,4-thiadiazole derivatives (Figure 1) have a great interest due to their promising biological and industrial applications.9–14
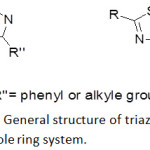 |
Figure 1: General structure of triazole and thiadiazole ring system. |
Thus, the synthesis and study of new 1,4-disubstituted 1,2,3-triazole and 2-amino-1,3,4-thiadiazole derivatives have been attracting the chemists over the recent years.15–17 Currently, the copper (I) catalysed azide-alkyne cycloaddition (CuAAC, also known as click reaction) that was introduced by Sharpless and et al in 2002 is the method choice for the synthesis of 1,4-disubstituted-1,2,3-triozle derivatives18-20 While for the synthesis of 2-amino-1,3,4-thiadiazole ring system, many approaches have been reported and most of them proceed via a cyclization reaction of the intermediates such as acylthiosemecarbazide.21–24 The combination of two or more pharmacophores in one molecule is a powerful strategy in the search for discovering new applications of heterocyclic compounds25. Thus, we planned to synthesize a series of new 1,4-disubstituted-1,2,3-triazole derivatives and then combine its 1,2,3-triazole ring system with 1,3,4-thiadiazole ring in one molecule to obtain a novel heterocyclic compounds. In the current work, the retrosynthetic pathway shows that 1,2,3-triazole and 1,3,4-thiadiazole rings are linked together directly via a covalent C-C bond at positions C4 and C5 of their structures, respectively.
Experimental
Chemicals and Instruments
All starting materials and solvents that are necessary for the synthesis of the target compounds were purchased from available sources and were directly used without further purification. FT-IR spectra were recorded on an FT-IR–8400S plus spectrometer operating from (4000–400 cm-1) as a KBr disc. 1H-NMR and 13C-NMR spectra were recorded at 500 MHz and 125 MHz, respectively on a Bruker AC400 spectrometer. The spectral data are mentioned using the following abbreviations for peak patterns: s-singlet, m-multiplet. The mass spectra (MS) were recorded using an Agilent 1100 Ion trap mass spectrometer operating at 70ev–. TG-DTG analysis was performed by using a Perkin Elmer TG 4000 at a temperature range 39-400°C in a heating rate 40°C/min under nitrogen.
Procedures
Synthesis of p-substituted Phenyl Azide Compounds (1-5)
All phenyl azide compounds that were used in the current work were synthesized according to the modified procedure that was described in the literature.26
General Procedure for the Synthesis of 1,2,3-Triazole Derivatives (6-10)
An aqueous solution of sodium ascorbate (0.8 g, 4.0 mmole was dissolved in water 2.0 ml) was added to a stirred aqueous solution containing CuSO4.5H2O (0.65 g, 3.0 mmole was dissolved in water 2.0 ml). To the resulting solution, a mixture of tert-butanol:water (15.0 ml 2:1), propiolic acid (2.1 ml, 30.0 mmole) and an appropriate p-substituted-phenyl azide 1-5 (30.0 mmole) were added, sequentially. The resulting reaction mixture was slowly heated up to 50°C and stirred for overnight. The reaction mixture was allowed to cool into room temperature before being treated with water (100 ml). The formed precipitate was collected by vacuum filtration, washed with diethyl ether and dried to obtain the target compounds as solid products.
Synthesis of 1-(4-methoxyphenyl)-1H-[1,2,3]-triazolyl-4-carboxylic acid 6
It was prepared by using compound 1 (4.47 g, 30.0 mmole): Yield (5.0 g, 75 %) as a brown solid. FT-IR (KBr disc, cm-1): 3293-2500 (OH, COOH), 3151 (CH-aromatic), 2930 (CH-aliphatic), 1730 (C=O), 1610 (C=C, triazole), 1564 (C=C, Ar) and 1437 (N=N). 1H-NMR (500 MHz, DMSO-d6): δ= 3.8 (s, 3H, OCH3), 7.1-7.8 (m, 4H, Ar-H) and 9.2 (s, 1H, C=CH, triazole ring). HPMS-EI+ (m/z): Calc. for C10H9N3O3 = 219.0, Found = 219.1.
Synthesis of 1-(4-methylphenyl)-1H-[1,2,3]-triazol-4-yl carboxylic acid 7
It was prepared by using compound 2 (3.9 g, 30.0 mmole): Yield (3.5 g, 50 %) as a pale brown solid. FT-IR (KBr disc, cm-1): 3332-2560 (-OH, COOH), 3134 (CH-aromatic), 2883 (CH-aliphatic), 1720 (C=O), 1640 (C=C, triazole), 1606 (C=C, Ar) and 1471 (N=N, triazole). 1H-NMR (500 MHz, DMSO-d6): δ= 2.3 (s, 3H, CH3), 7.3-7.7 (m, 4H, Ar-H) and 8.7 (s,1H, C=CH triazole). HPMS-EI+ (m/z): Calc. for C10H9N3O2 = 203.2, Found = 203.0.
Synthesis of 1-(4-hydroxyphenyl)-1H-1,2,3-triazoly-4-yl-carboxylic acid 8
It was prepared by using of compound 3 (4.0 g, 30.0 mmole): Yield (6.3 g, 76 %) as a black precipitate. FT-IR (KBr disc, cm-1): 3500-2800 (-OH, COOH), 3134 (CH-aromatic), 2970 (CH-aliphatic), 1727 (C=O), 1608 (C=C, triazole), 1573 (C=C, Ar) and 1470 (N=N). 1H-NMR (500 MHz, DMSO-d6): δ= 9.5 (s, 1H, OH-phenol ), 6.9- 7.7 (m, 4H, Ar-H) and 8.7 (s, 1H, C=CH, triazole). HPMS-EI+ (m/z): Calc. for C9H7N3O3 = 205.0, Found = 205.1.
Synthesis of 1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl carboxylic acid 9
It was prepared by using compound 4 (5.9 g, 30.0 mmole): Yield (7.5 g, 93 %) as a brown precipitate. FT-IR (KBr disc, cm-1): 3475-2400 (OH, COOH), 3028 (CH-aromatic), 2930 (CH-aliphatic), 1695 (C=O), 1614 (C=C, triazole), 1585 (C=C, Ar), 1402 (N=N) and 734 (C-Br). 1H-NMR (500 MHz, DMSO-d6): δ= 7-8.3 (m,4H, Ar-H), 8.9 (s, 1H, C=CH, triazole ring). HPMS-EI+ (m/z): Calc. for C9H6N3O2Br = 268.0, Found = 267.0.
Synthesis of 1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl carboxylic acid 10
It was prepared by using compound 5 (4.6 g, 30.0 mmole): Yield (6 g, 89 %) as a wight precipitate. FT-IR (KBr disc, cm-1): 3468-2570 (-OH, COOH), 3100 (CH-aromatic), 2852 (CH-aliphatic), 1702 (C=O), 1552 (C=C, triazole), 1500 (C=C, Ar) and 1431 (N=N). 1H-NMR (500 MHz, DMSO-d6): δ= 7.7-8.1 (m, 4H, Ar-H), 8.8 (s, 1H, C=CH, triazole ring). HPMS-EI+ (m/z): Calc. for C9H6N3O2Cl= 223.02, Found= 223.0.
General procedure for synthesis of 1,3,4-Thiadiazoles derivatives (11-15)
An appropriate 1,2,3-triazole derivative 6-10 (20.0 mmole) and thiosemicarbazide (1.8 g, 20.0 mmole) were dissolved in POCl3 (30.0 ml). The resulting reaction mixture was refluxed for 7.0 hours. The reaction mixture was then allowed to cool into room temperature before being slowly poured onto crush-ice (200 g). The resulting solution was stirred for 15 minutes before being treated carefully with an aqueous solution of sodium hydroxide 50%. The precipitate was then collected by filtration under vacuum, washed with water and dried to obtained the target compounds as solid products.
Synthesis of 2-amino-5-[1-(4-methoxy phenyl)-1H-1,2,3-triazol-4-yl]-1,3,4 thiadiazole 11
It was prepared by using compound 6 (4.3 g, 20.0 mmole): Yield (5.0 g, 92 %) as a dark green precipitate. FT-IR (KBr disc, cm-1): 3287-3130 (-NH2), 2962 (CH-aromatic), 2864 (CH-aliphatic), 1665 (-C=N-), 1612 (C=C, triazole), 1518 (C=C, Ar ) and 1440(-N=N). 1H-NMR (500 MHz, DMSO-d6): δ= 3.8 (s, 3H, OCH3), 8.1-8.3 (m, 4H, Ar-H) and 8.4 (s, 1H, C=CH, triazole ring). 13C-NMR (125 MHz, DMSO, d6): δ= 160, 140, 132, 130, 125, 123, 120, 115 and 56. HPMSEI+ (m/z): Calc. for C11H10N6SO = 274.0, Found = 274.1.
Synthesis of 2-amino-5-[1-(4-methylphenyl)-1H–1,2,3-triazol-4-yl]-1,3,4-thiadiazole 12
It was prepared by using compound 7 (4.0 g, 20.0 mmole): Yield (3.5 g, yield 78 %) as a brown precipitate. FT-IR (KBr disc, cm-1): 3414-3100 (-NH2), 3144 (CH-aromatic), 2937 (CH-aliphatic), 1647 (-C=N), 1610 (C=C, triazole), 1504 (C=C, Ar) and 1440(-N=N- triazole).1H-NMR (500 MHz, DMSO, d6) δ= 2.4 (s, 3H, CH3), 6.9-7.7 (m, 4H, Ar-H) and 9.7 (s, 1H, C=CH, triazole ring). 13C-NMR (125 MHz, DMSO-d6): δ= 165, 154, 147.5, 134, 132, 120, 115 and 110. HPMS-EI+ (m/z): Calc. for C11H10N6S = 258.0, Found = 258.0.
Synthesis of 2-amino-5-[1-(4-hydroxyphenyl)-1H-1,2,3-triazol-4-yl]-1,3,4-thiadiazole 13
It was prepared by using compound 8 (4.1 g, 20.0 mmole): Yield (4.6 g, 88 %) as a black precipitate. FT-IR (KBr disc, cm-1): 3445-3150 (-NH2), 3306 (-OH), 3010 (CH-aromatic), 2744 (CH-aliphatic), 1643 (-N=C-), 1606 (C=C, triazole) and 1512 (C=C, Ar). 1H-NMR (500 MHz, DMSO-d6): δ= 9.4 (s, 1H, OH- phenol), 7.3-8 (m, 4H, Ar-H) and 9.4 (s, 1H, C=CH, triazole). 13C-NMR (125 MHz, DMSO-d6): δ= 165, 152, 154.2, 133, 130, 120, 122.3 and 116.2. HPMS-EI+ (m/z): Calc. for C10H8N6SO = 260.0, Found = 260.0.
Synthesis of 2-amino-5-[1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl]-1,3,4-thiadiazole 14
It was prepared by using compound 9 (5.3 g, 20.0 mmole): Yield (5.5 g, 85 %) as a gray precipitate. FT-IR (KBr disc, cm-1): 3400-3320 (NH2), 3009 (CH-aromatic), 2901 (CH-aliphatic), 1653 (-N=C-), 1620 (C=C, triazole), 1552 (C=C, Ar) and 1420 (-N=N-). 1H-NMR (500 MHz, DMSO-d6): δ= 7.5-8 (m, 4H, Ar-H), 9.8 (s, 1H, C=CH, triazole ring). 13C-NMR (125 MHz, DMSO-d6): δ= 168, 160, 148, 142, 136, 133, 123 and 120. HPMS-EI+ (m/z): Calc. for C10H7N6SBr = 323.9, Found = 324.0.
Synthesis of 2-amino-5-[1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl]-1,3,4-thiadiazole 15
It was prepared by using the compound 10 (5.3 g, 20 mmole): Yield (5.0 g, 90 %) as a dark green precipitate. FT-IR (KBr disc, cm-1): 3466-3120 (NH2), 2936 (CH-aliphatic), 1676 (-N=C-), 1590 (C=C, triazole), 1502 (C=C, Ar) and 1417 (-N=N-). 1H-NMR (500 MHz, DMSO-d6): δ= 7.4-8.1 (m, 4H, Ar-H) and 9.3 (s, 1H, C=CH, triazole). 13C-NMR (125 MHz, DMSO-d6): δ= 161, 165, 152, 145, 135, 133, 123 and 120. HPMS-EI+ (m/z): Calc. for C10H7N6ClS = 278.7, Found = 278.0.
Results and Discussion
Chemistry
In the current project, a series of rich and poor-electron p-substituted phenyl azide compounds 1-5 was designed as an azido component for CuAAC reaction. Choosing of different substituted phenyl azides is to investigate the effects of a structural variation on their chemical reactivity towards click reaction and the thermal behavior of the synthesized heterocyclic compounds 6-15. The intermediates azide compounds 1-5 were synthesized via reaction of diazonium salts with sodium azide (Scheme 1). Having the target azido compounds 1-5, we turned our efforts to combine them with the commercially available propiolic acid under click reaction conditions. The synthesis route of 1-(4-substituted phenyl)-1H-1,2,3-triazol-4-yl-carboxylic acid derivatives 6-10 is illustrated in Scheme 1. This synthetic route involved addition of propiolic acid and appropriate azide 1-5 to a solution of tert-butanol:water (2:1 v/v) containing CuSO4.5H2O and sodium ascorbate. The latter is a reducing agent to generate in situ Cu (I) species that are required to perform click reaction.
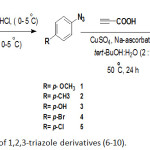 |
Scheme 1: Synthesis of 1,2,3-triazole derivatives (6-10). |
In this synthetic pathway, the reaction mixture was first left to stir at room temperature for overnight. However, the target compounds were obtained in a very low yield. An attempt to improve this low yield revealed that the standard click reaction solvent tert-butanol: water (1:1) is not enough to dissolve all amounts of the azido components 1-5. Once the ratio of the solvent tert-butanol:water was changed to (2:1 v/v) with heating up to 50 ̊C, the target compounds 6-10 were obtained in relatively high yields. Our synthesis route highlighted a simple work up and the products were pure enough without need to further purifications by column chromatography. The structures of the newly synthesized compounds were characterized by FT-IR, 1H-NMR and Mass spectra. Moreover, choosing of propiolic acid as an alkyne component in CuAAC reaction takes advantage that the synthesised 1,2,3-triazole 6-10 containing a carboxyl function group attached directly at C4 position of 1,2,3-triaozle ring system. So, it can be exploited to combine 1,2,3-triazole ring system with different structures. In the current work, we are interested in the combination of 1,2,3-triazole ring with 1,3,4-thiadiazole ring in one molecule. The most widely applicable route for the synthesis of 1,3,4-thiadiazole ring system is the thermal acid or base catalyzed dehydro-cyclization of their corresponding acylthiosemicarbazone to construct 2-amino-1,3,4-thiadiazole ring system.27,28 Therefore, the synthesised 1,2,3-triazole derivatives 6-10 and thiosemicarbazide were the key starting materials for the synthesis of the target compounds 11-15. The cyclocondensation of thiosemicarbazide with 1,2,3-triaozle derivatives compounds 6-10 in the presence of POCl3 under reflux conditions afforded series of new heterocyclic compounds containing 1,2,3-triazole and 1,3,4-thiadaizole ring systems combined in one molecule 11-15 as described in Scheme 2.
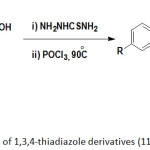 |
Scheme 2: Synthesis of 1,3,4-thiadiazole derivatives (11-15). |
Importantly, the work up of this reaction involved treating the resulting crude solutions with a solution of NaOH (50%) before being filtered. This step is necessary to remove any remaining unreacted amount of starting materials specially compounds 6-10, thereby leading to give the target compounds 11-15 are pure enough without any need to further purification by column chromatography. The structures of the newly synthesized compounds were characterized by FT-IR, 1H-NMR, 13C-NMR and Mass spectra.
Thermal stability study of the synthesised compounds (6-15)
Currently, thermal analysis has been become an important tool to study the thermal stability of important products such as organic compounds, polymers and pharmaceuticals.29 In the current study, the thermal stability studied by TG and DTG techniques. Thermogravimetry (TG) is one of the basic and useful analytical methods of thermal analysis that are used for recording weight lossing of a tested sample as a function of temperature or time in a heated environment.30 While, by DTG curve, the area of the peak (Tinitial, Tfinal) is directly proportional to the mass loss over the same temperature range and the DTG peak high at any temperature gives the rate of mass loss (dm/dT in mg/min unit).31,32 Therefore, the knowledge of the DTG curve helps to permit the direct application of the rate function of the change in the weight of the target sample to the study of phenomena occurring during thermogravimetric analysis. Practically, depending on the nature of the p-rich or poor-electron substituted phenyl that is attached to N1-position of 1,2,3-triazole ring of compounds 6, 7, 8, 9 and 10. TG-DTG measurements revealed that these compounds are thermally stable up to 250, 240, 275, 205 and 270°C, respectively. Followed a single step decomposition as shown in Figures 2, 3 and Table 1.
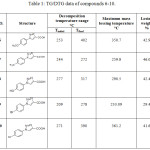 |
Table 1: TG/DTG data of compounds 6-10. |
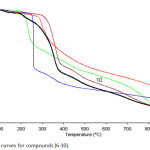 |
Figure 2: TG curves for compounds (6-10). |
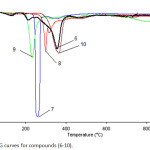 |
Figure 3: DTG curves for compounds (6-10). |
While, TG-DTG measurements that were recorded for compounds 11-15 indicted that these compounds are thermally behaved in the same manner that was described for their precursors 6-10. All compounds 11, 12, 13, 14 and 15 are thermally stable up to 260, 205, 279, 280 and 274 ̊C, respectively, followed with a single step decomposition as shown in Figures 4, 5 and Table 2.
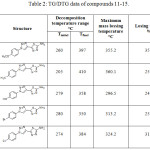 |
Table 2: TG/DTG data of compounds 11-15. |
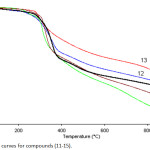 |
Figure 4: TG curves for compounds (11-15). |
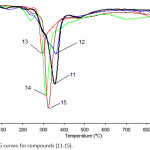 |
Figure 5: DTG curves for compounds (11-15). |
Conclusion
In conclusion, all poor-rich electron phenyl azide compounds were successfully reacted with propiolic acid under click reaction conditions. Since these compounds containing a carboxyl functional group, it used as a key to synthesise a series of new heterocyclic compounds containing 1,2,3-triazole and 1,3,4-thiadiazole rings in one molecule. Furthermore, all synthesized heterocyclic compounds displayed a relatively good thermal stability.
Acknowledgments
The authors would like to thank the department of chemistry/College of Science /Al Muthanna University for the facilities during doing this work.
Referances
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L. R.; Rodrigues, C. R.; Baptista, P. V.; Fernandes, A. R. Molecules. 2015. 20,(9), 16852-16891.
- Seeka, S.; Narsimha, S.; Jyostna, S.; Reddy, N.V. Int. J. Pharmacol. Pharm. Sci. 2015. 2,(4), 26-32.
- Singh, A. K.; Mishra, G.; Jyoti, K. Int. J. ChemTech Res. 2011. 1,(5), 44-49.
- Rajam, S.; StellaP, C. R.; Dileepan, G. B. J. Chem. Pharm. Res. 2016. 8,(5), 505-526.
- Yamamoto, T.; Muto, K.; Komiyama, M.; Canivet, J.; Yamaguchi, J.; Itami, K. Chem. Eur. J. 2011. 17,(36), 10113-10122.
- Otero, N.; Estévez, L.; Mandado, M.; Mosquera, R. A. Eur. J. Org. Chem. 2012. 10, (12), 2403-2413.
- Pawełczyk, A.; Sowa-Kasprzak, K.; Zaprutko, L. ECSOC. 2017. 1, 1-4.
- Jiang, J.; Zou, H.; Dong, Q.; Wang, R.; Lu, L.; Zhu, Y.; He, W. J. Org. Chem. 2016. 81, (1), 51-56.
- Rezki, N.; Al-Yahyawi, A. M.; Bardaweel, S. K.; Al-Blewi, F. F.; Aouad, M.R. Molecules. 2015. 20,(9), 16048-16067.
- Stefani, H. A.; Silva, N.C. S.; Manarin, F.; Lüdtke, D. S.; Zukerman-Schpector, J.; Madureira, L. S.; Tiekink, E. R. T. Tetrahedron Letters. 2012. 53, (14), 1742-1747.
- Hu, Y.; Li, C.; Wang, X.; Yang, Y.; Zhu, H. Chem. Rev. 2014, 114, (10), 5572-5610.
- Abdelriheem, NA.; Mohamed, AMM.; Abdelhamid, AO. Molecules. 2017, 22, (268), 1-16.
- Altintop, M.D.; Ozdemir, A.; Kucukoglu, K.; Turan-Zitouni, G.; Nadaroglu, H.; Kaplancikli, Z.A. J. Enzyme Inhib. Med. Chem. 2015, 30, (1), 32-37.
- Altintop, MD.; Can, ÖD.; Demir Özkay, Ü.; Kaplancikl, ZA. Molecules. 2016, 21, (8), 1-10.
- Hamama, S.W.; Gouda, M. A.; Abd El-Wahab, M. H.; Zoorob, H. H. J. Heterocyclic Chem. 2014, 51, 1558 -1581.
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. Drug Design, Development and Therapy. 2018, 12, 1545-1566.
- Kumar, A. S.; Ghule, V.D.; Subrahmanyam, S.; Sahoo, A. K. Chem. Eur. J. 2013, 19, (2), 509-518.
- Pengju, Ji.; Atherton, J. H.; I. Page, M. Org. Biomol. Chem. 2012, 10,(39), 7965-7969.
- Heravi, M. M.; Tamimi, M.; Yahyavi, H.; Hosseinnejad, T. Curr. Org. Chem. 2016, 20, (15), 1591-1647.
- Singh, M. S.; Chowdhury, S.; Koley, S. Tetrahedron. 2016, 72, (35), 5257-5283.
- El-Sadek, M. M.; Hassan, S.Y.; Abdelwahab, H.E.; Yacout, G. A. Molecules. 2012, 17, (7), 8378-8396.
- Hu, Y.; Li, C.Y.; Wang, X.; Yang, Y.; Zhu, H. Chem. Rev. 2014, 114, (10), 5572−5610.
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W. J. Org. Chem. 2015, 80, (2), 1018-1024.
- Parmar, KC.; Umrigar, N. H. J. Chem. Pharm. Res. 2017, 9, (6), 202-214.
- Kaserer, T.; Beck, K.R.; Akram, M.; Odermatt, A.; Schuster, D.; Willett, P. Molecules. 2015, 20, (12), 22799-22832.
- Lima-Neto, RG.; Cavalcante, NNM.; Srivastava, RM.; Mendonça Junior, F. J. B.; Wanderley, G. A. Molecules. 2012, 17, (5), 5882-5892.
- Manimaran, T.; Anand Raj, M.; Jishala, MI.; GopalasatheeskumarInt, K. J. Pharm. Anal. Res. 2017, 6, (2), 222-231.
- Al-Amiery, A. A.; Musa, A. Y.; Kadhum, A. A. H.; Mohamad, A. B. Molecules. 2011, 16, (8), 6833-6843.
- Abd El-Halim, H. F.; Omar, MM.; Mohamed, G. G. Spectrochim. Acta A. 2011, 78, (1), 36-44.
- Feist, M. ChemTexts. 2015, 1, (8), 1-12.
- Feist, M.; Teinz, K.; Manuel, SR.; Kemnitz, E. Thermochimica Acta. 2011, 524, 170-178.
- Rostek, E.; Biernat, K. J.Sustainable Develop. Energy, Water and Environ. Systems. 2013, 1, (2), 163-171.

This work is licensed under a Creative Commons Attribution 4.0 International License.









