Synthesis, Characterization and Anticonvulsant Activity of Novel Fused 1,2,4-Triazolo-1,3,4-Thiadiazoles
Mohammad Sarafroz*1, Yasmin Khatoon2, Niyaz Ahmad3, Mohammad Amir4, Salahuddin5 and Faheem Hyder Pottoo6
1Department of Pharmaceutical Chemistry, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, City Dammam, Saudi Arabia.
2School of Pharmacy, Department of Pharmaceutical Chemistry, Sharda University, Knowledge Park III, Greater Noida, Uttar Pradesh, India
3Department of Pharmaceutics, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, City Dammam, Saudi Arabia.
4Department of Pharmacognosy and Phytochemistry, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, City Dammam, Saudi Arabia.
5Department of Pharmaceutical Chemistry, Noida institute of Engineering and Technology (Pharmacy Institute), Greater Noida, Uttar Pradesh, India.
6Department of Pharmacology, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, City Dammam, Saudi Arabia.
Corresponding Author E-mail: drsarafroz@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350107
Article Received on : 01-10-2018
Article Accepted on : 01-02-2019
Article Published : 22 Feb 2019
A novel category of 1,2,4-triazolo-1,3,4-thiadiazoles were ready by the utilization of 3-amino-4-hydroxybenzoate as the beginning material. Spectral information results were used for the establishing of prepared compounds. Compounds were screened anticonvulsant activity for obtaining better results by MES test and scPTZ methods. The rotarod method was used for neurotoxicity analysis. Majority of the compounds displayed distinguished anticonvulsant impact practically identical to standard drugs (phenytoin and carbamazepine) with slight neurotoxicity.
KEYWORDS:Anticonvulsant; Neurotoxicity and Lipophilicity; Substituted Triazolo-Thiadiazole
Download this article as:| Copy the following to cite this article: Sarafroz M, Khatoon Y, Ahmad N, Amir M, Salahuddin S, Pottoo F. H. Synthesis, Characterization and Anticonvulsant Activity of Novel Fused 1,2,4-Triazolo-1,3,4-Thiadiazoles. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Sarafroz M, Khatoon Y, Ahmad N, Amir M, Salahuddin S, Pottoo F. H. Synthesis, Characterization and Anticonvulsant Activity of Novel Fused 1,2,4-Triazolo-1,3,4-Thiadiazoles. Orient J Chem 2019;35(1). Available from: https://bit.ly/2GXIWI6 |
Introduction
Convulsion is a condition of brain described via the irregular and unreliable episode of seizures. It is a collective neurological disorder, affecting 0.5-1% of the people globally according to epidemiological studies.1-3 Each year around 0.25 million new cases are added to this figures.4-5 More than twenty antiepileptic drugs of different classes (like carbamazepine, phenytoin, valproate, phenobarbital, vigabatrin, lamotrigine, tiagabine, topiramate, levetiracetam and gabapentin) are available for the treatment of seizures.6 Despite introduction of these novel AEDs, the management of epilepsy quiet insufficient and part of the patients suffers from several complications like neurotoxicity; symptoms of depression, CNS associated disorders, gingival hypertrophy, liver toxicity and megaloblastic anemias.7-8 In adequacy and toxicities are the restrictions of the present drugs.9-10 Because of these restrictions there is a critical need to create new antiepileptic agents with increase seizure control, increased acceptability, improved protection and pharmacokinetic properties with neuroprotective actions.11
In present times, an interest of fused triazolo-thiadiazole derivatives has received impressive consideration because of their biological significance. Compounds having triazole or thiadiazole as a core moiety are represented for wide range of pharmacological properties for example anticancer,12 anti-inflammatory,13 analgesic,14 antimicrobial,15 antibacterial,16 antifungal,17 insecticidal,18 herbicidal19 and CNS stimulating.20 Fused triazolo-thiadiazole derivatives were found to diverse biological activities for example, anti-inflammatory,21-23 antimicrobial24-25 and anticancer.26-27 Triazolo-thiadiazole, a heterocyclic compound of diverse biological activities was observed to be one of the novel classes of anticonvulsants as discovered by literature study.28 Recently, the area of antiepileptic drug development (ADD) has turned out to be very unique, bearing numerous promising examination openings, and is proceeding with interest for new chemical entities because it is difficult to manage each sort of seizure with existing medications.
Literature review reveals that substituted triazolo-thiadiazoles were not given careful thought for antiepileptic drug actions. Therefore, it was thought to plan and synthesize a combination of 1,2,4-triazolo-1,3,4-thiadiazoles as a fundamental core joined with substituted benzoxazole moiety within a single frame. Such combination is would have to make compounds with lipophilic character having promising anticonvulsant actions.
Materials and Methods
Chemistry
The liquid paraffin bath was used for determination of melting points (oC) and was uncorrected. The laboratory solvents were purchased from Spectrochem, C.D.H and S.D. Fine chem. Ltd. The different solvent systems were used for detection of completion of chemical reactions through TLC method. The iodine and UV light chamber were utilized for imagining of spots. The 1H-NMR spectrums were noted on Bruker 300 MHz and 400 Ultra ShieldTM spectrometer in DMSO/d6 and values are expressed in ppm in respect to tetramethylsilane. The FT-IR spectrophotometer (BIO-RAD FTS) was used for IR bands utilizing KBr pellets. UPLC-MS/MS spectrometer (WATERS, Mass Lynx version 4.1) was used for mass spectrum and elemental analysis was performed using Perkin-Elmer 2400 and found within ±0.4% of theoretical values.
Synthesis of Benzoxazole Hydrazide (2)
These compounds were obtained from methyl 3-amino-4-hydroxybenzoate by the outline described in the literature.30-31
Synthesis of Compound (3)
Equimolar quantities of acid hydrazide (2) and carbon disulfide were stirred for 12 hr in presence of 50 ml alcoholic potassium hydroxide solution at room temperature. Finally the reaction mixtures were cooled and poured onto ice. The potassium dithiocarbazinate, which isolated out, was separated, washed with ether and dried.28,30
Synthesis of Compound (4)
In this reaction compound (3) and hydrazine hydrate (1:2) was refluxed for 10-15 hr in water (50 mL). The reaction materials were cooled and acidify with hydrochloric acid. The resultant solid precipitate of 1,2,4-triazole separated out, splashed with ice water, desiccated and recrystallized from ethanol to give 4 in good yield.28,31
General Protocol for the Synthesis of 1,2,4-Triazolo-1,3,4-Thiadiazoles (5a-L)
The compound (4) and different substituted/unsubstituted aryl acids (1:1) in 10 ml of phosphoryl chloride was condensed for 5-6 hr. successively the contents were stirred on ice cold water for 4-5 hr. The content was permitted to stand overnight, the precipitate separated, neutralized with aqueous alkali, dehydrated and purified from ethanol.28,32
Compound (5a)
Yield 80%; m.p. 145-147°C; IR (KBr, cm-1): 3033 (CH str), 1611 (C=N, cyclic), 1480 (C-N, cyclic), 1227 (N-N=C, cyclic), 722 (C-Cl), 711 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.00-8.11 (m, 11H-Aromatic), 3.86 (s, 3H, OCH3). Elemental Analysis (C23H14ClN5O2S): Calcd. C, 59.83; H, 3.01; N, 14.98; Found: C, 60.07; H, 3.07; N, 15.23.
Compound (5b)
Yield 81%; m.p. 165-167°C; IR (KBr, cm-1): 3032 (CH str), 1641 (C=N, cyclic), 1492 (C-N, cyclic), 1218 (N-N=C, cyclic), 741 (C-Cl), 701 (C-S-C); 1H-NMR (DMSO-d6) δ (ppm): 7.34-8.19 (complex, m, 16H-Aromatic). Mass (m/z): 505 (M+1); Elemental Analysis (C28H16ClN5OS): Calcd. C, 66.65; H, 3.46:, N, 14.12; Found: C, 66.47; H, 3.19; N, 13.84.
Compound (5c)
Yield 56%; m.p. 155-157 °C; IR (KBr, cm-1): 3023 (CH str), 1655 (C=N, cyclic), 1475 (C-N, cyclic), 1214 (N-N=C, cyclic), 766 (C-Cl), 698 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.15-7.99 (complex, m, 17H-Aromatic), 5.11 (s, 1H CH, diphenyl). Elemental Analysis (C28H16ClN5OS): Calcd. C, 66.65; H, 3.46; N, 14.12; Found: C, 66.47; H, 3.19; N, 13.84.
Compound (5d)
Yield 70%; m.p. 120-122°C; IR (KBr, cm-1): 3036 (CH str), 1591 (C=N, cyclic), 1478 (C-N, cyclic), 1239 (N-N=C, cyclic), 723 (C-Cl), 717 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 6.90-7.79 (m, 12H-Aromatic), 5.06 (s, 2H, OCH2); Mass (m/z): 459 (M+1); Elemental Analysis (C23H14ClN5O2S): Calcd. C, 59.58; H, 3.43; N, 14.91; Found: C, 60.07; H, 3.07; N, 15.23.
Compound (5e)
Yield 72%; m.p. 195-197°C; IR (KBr, cm-1): 3065 (CH str), 1733 (carbonyl), 1611 (C=N, cyclic), 1489 (C-N, cyclic), 1223 (N-N=C, cyclic), 720 (C-Cl), 711 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 10.11 (s, 1H, CHO), 7.29-8.08 (m, 11H-Aromatic), 5.08 (s, 2H, OCH2); Elemental Analysis (C24H14ClN5O3S): Calcd. C, 59.24; H, 3.08; N, 13.99; Found: C, 59.08; H, 2.89; N, 14.35.
Compound (5f)
Yield 75%; m.p. 160-162°C; IR (KBr, cm-1): 3033 (CH str), 1738 (carbonyl), 1623 (C=N, cyclic), 1490 (C-N, cyclic), 1223 (N-N=C, cyclic), 724 (C-Cl), 692 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 10.27 (s, 1H, CHO), 7.36-8.01 (m, 11H-Aromatic); Mass (m/z): 457 (M+1); Elemental Analysis (C23H12ClN5O2S): Calcd. C, 60.41; H, 2.23; N, 15.11; Found: C, 60.33; H, 2.64; N, 15.29.
Compound (5g)
Yield 80%; m.p. 140-142°C; IR (KBr, cm-1): 3020 (CH str), 1611 (C=N, cyclic), 1498 (C-N, cyclic), 1256 (N-N=C, cyclic), 757 (C-Cl), 698 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.39-8.17 (m, complex, 11H-Aromatic); Elemental Analysis (C21H11ClN6OS): Calcd. C, 58.13; H, 3.01; N, 19.11; Found: C, 58.54; H, 2.57; N, 19.50.
Compound (5h)
Yield 63%; m.p. 150-152°C; IR (KBr, cm-1): 3037 (CH str), 1601 (C=N, cyclic), 1484 (C-N, cyclic), 1244 (N-N=C, cyclic), 714 (C-Cl), 700 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.10-7.98 (m, 12H-Aromatic), 3.48 (2H, m, CH2); Elemental Analysis (C23H14ClN5OS): Calcd. C, 62.10; H, 3.15; N, 15.80; Found: C, 62.23; H, 3.18; N, 15.78.
Compound (5i)
Yield 60%; m.p. 180-182°C; IR (KBr, cm-1): 3576 (OH str), 2998 (CH str), 1608 (C=N, cyclic), 1490 (C-N, cyclic), 1235 (N-N=C), 734 (C-Cl), 701 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 1078 (s, 3H, 3×OH), 6.86-8.98 (m, 9H-Aromatic); Mass (m/z): 477 (M+1); Elemental Analysis (C22H12ClN5O4S): Calcd. C, 55.01; H, 2.44; N, 14.83; Found: C, 55.29; H, 2.53; N, 14.66.
Compound (5j)
Yield 67%; m.p. 175-177°C; IR (KBr, cm-1): 2996 (CH str), 1604 (C=N, cyclic), 1489 (C-N, cyclic), 1220 (N-N=C), 719 (C-Cl), 697 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.30-8.11 (m, complex, 14H-Aromatic), 4.38 (s, 2H, CH2); Mass (m/z): 493 (M+1); Elemental Analysis (C27H16ClN5OS): Calcd. C, 65.71; H, 3.12; N, 14.30; Found: C, 65.65; H, 3.26; N, 14.10.
Compound (5k)
Yield 51%; m.p. 165-167°C; IR (KBr, cm-1): 3564 (OH str), 3004 (CH str), 1603 (C=N, cyclic), 1474 (C-N, cyclic), 1214 (N-N=C), 733 (C-Cl), 691 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 10.11 (s, 1H, OH), 6.92-7.83 (m, 11H-Aromatic); Elemental Analysis (C22H12ClN5O2S): Calcd. C, 58.91; H, 3.01; N, 15.83; Found: C, 59.26; H, 2.71; N, 15.71.
Compound (5l)
Yield 62%; m.p. 155-157°C; IR (KBr, cm-1): 3033 (CH str), 1730 (O-C=O), 1583 (C=N, cyclic), 1463 (C-N, cyclic), 1260 (N-N=C), 690 (C-S-C, cyclic); 1H-NMR (DMSO-d6) δ (ppm): 7.06-8.07 (m, 11H-Aromatic), 2.48 (s, 3H, CH3); Elemental Analysis (C24H14ClN5O3S): Calcd. C, 58.88; H, 3.01; N, 13.98; Found: C, 59.08; H, 2.89; N, 14.35.
Pharmacological Screening
The anticonvulsant screening of the final compounds (5a-l) were tested on Swiss albino mice (20-25 g), permitted by the Institutional Animal Ethics committee, R.V. Northland Institute, Dadri, Greater Noida, Uttar Pradesh-India, under the proposal number RVNI/IAEC/2017/05. The method was followed conferring to the Antiepileptic Drug Development Program33-34. All the newly synthesized compounds (5a-l) were suspended in polyethylene glycol (PEG-400) and administered intraperitoneally.
The Maximal Electroshock Seizure Test
This method uses a current intensity of 50mA, 60Hz, connected by means of corneal terminals for 0.2 s for induction of seizures. The maximal seizure consistently involves a little time of tonic extension of the hind limbs and a last clonic scene. Absence of even a threshold seizure was noted as an estimation of antiseizure action.34
The scPTZ Seizure Test
The assessment method was taken after with reference to the known protocol.35 This procedure uses 85 mg/kg of scPTZ that causes attacks in >97% of the mice. The experimental candidates were administered half an hour before the scPTZ; the defend was identified in relations of absence of a scene of clonic spasm for 5 sec duration showed a candidate’s capacity to nullify the impact of PTZ happening seizure edge.
Minimal Motor Impairment (Neurotoxicity) Screening
The technique was taken after with reference to the known protocol.36 The mice could remain on a fast-tracking rod (10 rpm, 3.2cm diameter). Those group of mice were used for motor impairment study, which could remain proceeding the spinning pole for not less than one min. Motor incapacity was appeared by the disappointment of the mice to keep up adjust on the bar for not less than 1 min.
Lipophilicity Determination
The calculation method was taken after with reference to the known protocol.38 The congener acts on CNS were associated with lipophilicity37 and it was revealed that those medications which follow up on focal sensory system, having an ideal lipophilic character (log P ≈ 2). Here we endeavored to relate the antiepileptic action of the titled compounds with their figured log P standards.
Result and Discussion
A novel class of 1,2,4-triazolo-1,3,4-thiadiazoles were prepared in appropriate yields as demonstrated in scheme 1 and the compounds were confirmed through elemental and spectral results, are described in experimental procedures. The starting material substituted benzoate are cyclized to substituted benzoxazole carboxylate (1) on treatment with 3-chlorobenzoic acid in alcohol, which on treatment with hydrazine hydrate, afforded acid hydrazide (2).29,30 Potassium dithiocarbazinate (3) was prepared in alcoholic KOH with CS2,28,29 which on additionally condensed with hydrazine to afforded substituted benzoxazole mercapto-traiazole (4) through direct hydrazinolysis method,28,39 which was condensed with different acids in phosphoryl chloride to yield the titled compounds 1,2,4-triazolo-1,3,4-thiadiazoles (5a-l). The structure of compound (4) was declared by 1H-NMR spectral results. The 1H-NMR spectra demonstrated that the -SH moiety appeared as singlet at δ 13.21-13.62, while the -NH2 moiety showed up as a singlet at δ 5.44-5.62. Although in the 1H-NMR spectra of triazolo-thiadiazoles the indications of -NH2 and –SH protons disappeared, affirming that triazole was converted into triazolo-thiadiazoles by reaction with different aromatic acid derivatives.
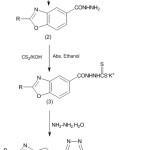 |
Scheme 1: Synthesis of novel triazolo-thiadiazoles. |
The anticonvulsant activity of the final compounds was examined by the traditional method given by the epilepsy division of the NINDS conferring to the Antiepileptic Drug Development program.33-35 Moreover, intense lethality of antiepileptic medicates in rodents is constantly showed by neurological deficits can be identified by the rotarod test.36
Table 1: Physical date of the titled molecules (5a-l).
| Compound | R | R1 | Mol. formulaa | m.p b (°C) | Log Pc | Rfd Value |
| 5a | 3-ClC6H4 | 4-OCH3C6H4 | C23H14ClN5O2S | 145-146 | 1.88 | 0.80 |
| 5b | 3-ClC6H4 | 4- C6H5C6H4 | C28H16ClN5OS | 165-166 | 1.92 | 0.81 |
| 5c | 3-ClC6H4 | 2- C6H5C6H5CH | C28H16ClN5OS | 155-157 | 0.98 | 0.61 |
| 5d | 3-ClC6H4 | C6H5OCH2 | C23H14ClN5O2S | 110-112 | 1.90 | 0.70 |
| 5e | 3-ClC6H4 | 4-CHOC6H4OCH2 | C24H14ClN5O3S | 195-196 | 2.18 | 0.70 |
| 5f | 3-ClC6H4 | 4-CHOC6H4 | C23H12ClN5O2S | 160-162 | 1.87 | 0.75 |
| 5g | 3-ClC6H4 | C5H4N | C21H11ClN6OS | 140-142 | 2.11 | 0.80 |
| 5h | 3-ClC6H4 | C6H5CH2 | C23H14ClN5OS | 150-151 | 1.03 | 0.63 |
| 5i | 3-ClC6H4 | 3,4,6-OH-C6H2 | C22H12ClN5O4S | 180-182 | 2.19 | 0.60 |
| 5j | 3-ClC6H4 | C10H7CH2 | C27H16ClN5OS | 140-142 | 1.09 | 0.82 |
| 5k | 3-ClC6H4 | 2-OH C6H4 | C22H12ClN5O2S | 175-176 | 1.56 | 0.81 |
| 5l | 3-ClC6H4 | 2-OCOCH3C6H4 | C24H14ClN5O3S | 155-156 | 1.89 | 0.62 |
aSolvent of crystallization ‒ ethanol.
bm.p of the compounds at their dissolution.
cLog P was calculated using absorbance data, chloroform / phosphate buffer at 28°C.
dSolvent system – benzene :acetone (8:2, v/v), benzene:ethanol (2:0.5, v/v), toluene:ethylacetate:formic acid (5:4:1, v/v/v).
Table 2: Anticonvulsant and neurotoxicity screening of the titled molecules (5a-l).
|
Compound |
Intraperitoneal dose in micea | Neurotoxicity screena | ||||
| MES screen | scPTZ screen | |||||
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h |
4 h |
|
| 5a | 100 | 300 | — | — | 300 | — |
| 5b | 100 | — | 300 | — | — | 300 |
| 5c |
— |
— |
— |
— |
× | × |
| 5d | 100 |
300 |
— |
300 |
300 |
— |
| 5e | 30 |
300 |
300 | — | — | 300 |
| 5f | 100 |
300 |
300 | 300 | 300 | — |
| 5g | 30 |
300 |
— | 300 | — | — |
| 5h | — | — | — | — | × | × |
| 5i | 30 | 300 | — | 300 | — | — |
| 5j | — | 300 | — | — | 300 | — |
| 5k | 300 | — | — | — | — | — |
| 5l | 100 | 300 | — | — | 300 | — |
| Phenytoinb | 30 | 30 | — | — | 100 | 100 |
| Carbamazepineb | 30 | 100 | 100 |
300 |
300 |
300 |
aDoses of 30, 100 and 300 mg/kg were managed to mice via i.p. route. The mice were observed 0.5 and 4 hrs after the treatment. The trace (—) specifies nonappearance of activity at higher dose (300 mg/kg) and cross (×) means not screened. Propylene glycol (0.1 ml, i.p.) was utilized as controller..
bData of standard drugs (phenytoin and carbamazepine) were gotten referring.40
In the anticonvulsant activity, each compound showed action except for 5c and 5h. Compound 5e, 5g and 5i were seen to be extremely powerful at dosage of 30 mg/kg against the MES method at 0.5 h time intervals characteristic for their capacity to maintain a strategic distance from seizure spread at by and large cut down dosage. Compounds viz. 5a, 5b, 5d, 5f and 5l that indicated protection at a moderate level compared to MES model at dosage of 100 mg/kg. The activity of molecules like 5a, 5d, 5e, 5f, 5g, 5i and 5l indicated at both time intervals. In this way, the larger part of the molecules exhibited anticonvulsant activities at 0.5 h interim having a quick beginning and smaller span of movement. In scPTZ examination, every compound except 5a, 5c, 5h, 5j, 5k and 5l demonstrated movement characteristic of their capacity to counteract seizure attack. Compounds 5b and 5e indicated 100% safety at a dosage of 300 mg/kg at 0.5 h and have rapid beginning however for short term of activity. A couple of compounds like 5d, 5g and 5i were likewise dynamic after 4.0 hrs expanded time of activity. Just a single compound (5f) established activity at higher dose (300 mg/kg) on both time intervals. In neurotoxicity screening, rotarod test was utilized to measure the undesired impacts of the synthesized compounds, like sedation and ataxia. Compounds 5g, 5i and 5k showed no any mortality at the higher dose (300 mg/kg). No any compounds were lethal at 0.5 and 4.0 hrs. however compounds 5a, 5d, 5f, 5j and 5l demonstrated lethality after 0.5 h and don’t indicate harmfulness after 4.0 hrs. Only two compounds (5b and 5e) exhibited delayed toxicity simply after 4.0 hrs, is practically identical of standard drug carbamazepine (300 mg/kg). Though, every molecule was less harmful than standard drug phenytoin (100 mg/kg). The compounds 5a, 5b, 5d, 5e, 5f, 5g, 5i and 5l identified as additional hydrophobic character having strong anticonvulsant action. Compound 5j and 5k were also hydrophobic having little intensity. Only two compounds (5c and 5h) were a lesser amount of lipophilicity and were not dynamic in MES and scPTZ tests.
Conclusion
In the present investigation, novel 1,2,4-triazolo-1,3,4-thiadiazoles was successfully prepared and estimated for anticonvulsant activity by MES and scPTZ tests. Entire compounds displayed moderate to good activity. Preliminary evaluation indicates the target compounds 5e, 5g and 5i exhibited potent anticonvulsant activity at a lower dosage (30 mg/kg). The molecules like 5a, 5d, 5e, 5f, 5g, 5i and 5l exhibited activity at 0.5 and 4.0 hrs in contrast to seizures it may would-be worth as prototypic candidates. The anticonvulsant data shown that every compound showed distinctive decrease of hind limb tonic extensor stage. Moreover, anticonvulsant activities of the other tested compounds were found to be much less effective than standard drugs (phenytoin and carbamazepine). According to the results obtained it seems that presence of halo-substituted aryl at benzoxazole and hydroxyl and aldehyde substituted aryl at triazolo-thiadiazole moiety displayed the best anticonvulsant activity and favorable high protection. Compounds 5a, 5b, 5d, 5e, 5f, 5g, 5i and 5l supposedly were more lipophilic character having strong anticonvulsant activity. Compounds 5j and 5k were a lesser amount of lipophilicity and a reduced amount of activities in MES test. Subsequently, triazolo-thiadiazoles were found having anticonvulsant properties, and express to a favorable candidates with fascinating pharmacological values.
Acknowledgements
Authors are sincerely thanks to IIT Delhi and Jamia Hamdard University for spectral analysis of the compounds. We extend our sincere thanks to the Antiepileptic Drug Development program for completing the anticonvulsant test.
Conflict of Interest
There is no conflict of interest.
References
- Njamnshi, A. K.; Bissek, A. C. Z. K.; Yepnjio, F. N.; Tabah, E. N.; Angwafor, S. A.; Kuate, C. T.; Dema, F.; Fonsah, J. Y.; Acho, A.; Kepeden, M. N.; Azinwi, Y. H.; Kuwoh, P. B,; Angwafor, F. F.; Muna, W. F. Epi. Behav. 2010, 17, 95-102.
- Bell, G. S.; Sander, J. W. Seizure, 2002, 11(Suppl.A), 306-314.
- Husain, A.; Siddiqui, N.; Sarafroz, M.; Khatoon, Y.; Rasid, M.; Ahmad, N. Acta. Pol. Pharma. Drug Res. 2011, 68, 657-663.
- Siddiqui, N.; Pandeya, S. N.; Khan, S. A.; Stables, J.; Rana, A.; Alam, M. Bioorg. Med. Chem. Lett. 2007, 17, 255-259.
- Sabers, A.; Gram, L. Drugs. 2000, 60, 23-33.
- White, H. S. Epilepsia. 2003, 44, 2-8.
- Belcastro, V.; Striano, P.; Gorgone, G.; Costa, C.; Ciampa, C.; Caccamo, D.; Pisani, L. R.; Oteri, G.; Marciani, M. G.; Aguglia, U.; Striano, S.; Ientile, R.; Calabresi, P.; Pisani, F. Epilepsia. 2010, 51, 274-279.
- Meador, K. J. J. Clin. Psychi. 2003, 64(Suppl.8), 30-34.
- Leppik, I. E. Epilepsia. 1994, 35, 29-40.
- Brodie, M. Lancet. 1992, 339, 1397-1400.
- Szelenyi, I.; Horvath, K.; Howes, J. F.; Mazarat, A. M. Drugs Fut. 2003, 28, 925-936.
- Li, Y.; Liang, J.; Siu, T.; Hu, E.; Rossi, M. A.; Barnett, S. F.; Defeo-Jones, D.; Jones, R. E.; Robinson, R. G.; Leander, K.; Huber, H. E.; Mittal, S.; Cosford, N.; Prasit, P. Bioorg. Med. Chem. Lett. 2009, 19, 834-836.
- Kumar, H.; Javed, S. A.; Khan, S. A.; Amir, M. Eur. J. Med. Chem. 2008, 43, 2688-2698.
- Salgın-Goksen, U.; Gokhan-Kelekci, N.; Goktas, O.; Koysal, Y.; Kilic, E.; Isik, S.; Aktay, G.; Ozalp, M. Bioorg. Med. Chem. 2007, 15, 5738-5751.
- Gumrukcuoglu, N.; Bekircan, O.; Serdar, M.; Celik, E.; Sevim, A.; Demirbas, N. Turk. J. Chem, 2007, 31, 335-348.
- Guzeldemirci, N. U.; Kucukbasmaci, O. Eur. J. Med. Chem. 2010, 4, 63-68.
- Siddiqui, A. A.; Arora, A.; Siddiqui, N.; Misra, A. Ind. J. Chem. 2005, 44, 838-841.
- Chai, B.; Qian, X.; Cao, S.; Liu, H.; Song, G. Arkivoc. 2003, 2, 141-145.
- Ma, Y.; Liu, R.; Gong, X.; Li, Z.; Huang, Q.; Wang, H.; Song, G. G. J. Agri. Food. Chem. 2006, 54, 7724-7728.
- Nagai, S. I.; Ueda, T.; Sugiura, S.; Nagatsu, A.; Murakami, N.; Sakakibara, J.; Fujita, M.; Hotta, Y. J. Het. Chem. 1998, 35, 325-327.
- El-Shehry, M. F.; Abu-Hashem, A. A.; El-Telbani, E. M. Eur. J. Med. Chem. 2010, 45, 1906-1911.
- Metwally, K. A.; Yaseen, S. H.; Lashine, E. S. M.; El-Fayomi, H. M.; El-Sadek, M. E. Eur. J. Med. Chem. 2007, 42, 152-160.
- Amir, M.; Kumar, H.; Javed, S. A. Eur. J. Med. Chem. 2008, 43, 2056-2066.
- Sahu, J. K.; Ganguly, S.; Kaushik, A. J. Adv. Pharma. Tech. Res. 2014, 5(2), 90-95.
- Karegoudar, P.; Prasad, D. J.; Ashok, M.; Mahalinga, M.; Poojary, B.; Holla, B. S. Eur. J. Med. Chem. 2008, 43(4), 808-815.
- Ramaprasad, G. C.; Kalluraya, B.; Kumar, B. S.; Mallya, S. Der Pharma. Chemi. 2012, 4(3), 1026-1032.
- Sunil D, Isloor AM, Shetty P. Synthesis, characterization and anticancer activity of 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles on Hep G2 cell lines. Der Pharma Chemi, 2009; 1(2): 19-26.
- Husain, A.; Naseer, M. A.; Sarafroz, M. Acta Pol. Pharma. Drug Res. 2009, 66, 135-140.
- Plech, T.; Wujec, M.; Kosikowska, U.; Malm, A.; Kapro, B. Eur. J. Med. Chem. 2012, 47, 580-584.
- Gopalakrishna, B.; Ranghunandan, N.; Rao, V. J.; Bari, S.; Venkatesham, A.; Sarangapani, M. Ind. Drug. 2005, 5, 369-374.
- El-Shehry, M. F.; Abu-Hashem, A. A.; El-Telbani, E. M. Eur. J. Med. Chem. 2010, 45, 1906-1911.
- Li, Y. J.; Liu, L. J.; Jin, K.; Xu, Y. T.; Sun, S. Q. Chin. Chem. Lett. 2010, 21, 293-296.
- Porter, R. J.; Cereghino, J. J.; Gladding, G. D.; Hessie, B. J.; Kupferberg, H. J.; Scoville, B.; White, B. G. Clev. Clin. Qua. 1984, 51, 293-305.
- Krall, R. L.; Penry, J. K.; White, B. G.; Kupferberg, H. J.; Swinyard, E. A. Epilepsia, 1978, 19, 409-428.
- Swinyard, E. A.; Woodhead, J. H.; White, H. S.; Franklin, M. R.; Levy, R. H.; Mattson, R. H.; Melrum, B.; Penry, J. K.; Dreifuss, F. E. Raven-Press, 85-102.
- Dunham, N, W.; Miya, T. S. J. Ame. Pharma. Asso. 1957, 46, 208-209.
- Lien, E. J.; Liuo, R. C. H.; Shinoucla, H. G. J. Pharma. Sci. 1979, 68, 463-468.
- Farrar, V. A.; Ciechanowicz-Rutkowska, M.; Grochowski, J.; Serda, P.; Pilati, T.; Filippini, G.; Hinko, C. N.; El-Assadi, A.; Moore, J. A.; Edafiogho, I. O.; Andrews, C. W.; Cory, M.; Nicholson, J. M.; Scott, K. R. J. Med. Chem. 1993, 36, 3517-3525.
- Reid, J. R.; Heindel, N. D. J. Heterocy. Chem. 1976, 13, 925-926.
- Dimmock, J. R.; Vashishtha, S. C.; Stables, J. P. Pharmazie. 2000b, 55, 490-494.

This work is licensed under a Creative Commons Attribution 4.0 International License.









