Synthesis and Antibacterial Screening of Novel Thiazolyl Pyrazole and Benzoxazole
Vaibhav Prabhakar Landage1, Dilip Raosaheb Thube1 and Bhausaheb Kisan Karale2
1Department of Chemistry, New Arts, Commerce and Science College, Parner, Ahmednagar-414302, India, Affiliated to the Savitribai Phule Pune University, Pune, India.
2Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar -414001, India, Affiliated to the Savitribai Phule Pune University, Pune, India.
Corresponding Author E-mail: drthube@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/350164
Article Received on : 18-06-2018
Article Accepted on : 10-12-2018
Article Published : 11 Jan 2019
A new series of (2-hydroxyphenyl)(1-(4-p-tolylthiazol-2-yl)-1H-pyrazol-4-yl)methanone 3a-g, 2[(E)–{1-[4-(p-tolyl)-1, 3-thiazol-2-yl)]-1H-pyrazol-4-yl} (hydroxyimino) methyl] phenol 4a-g and 2-(1-(4-p-tolylthiazol-2-yl)-1H-pyrazole-4-yl)benzo[d]oxazole 5a-g have been synthesised. These synthesised compounds have been characterised by the spectral, analytical data and scanned for their antibacterial activities.
KEYWORDS:Benzo[d]oxazole; 3-formylchromone; Pyrazole; Thiazole
Download this article as:| Copy the following to cite this article: Landage V. P, Thube D. R, Karale B. K. Synthesis and Antibacterial Screening of Novel Thiazolyl Pyrazole and Benzoxazole. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Landage V. P, Thube D. R, Karale B. K. Synthesis and Antibacterial Screening of Novel Thiazolyl Pyrazole and Benzoxazole. Orient J Chem 2019;35(1). Available from: https://bit.ly/2E0Vtsp |
Introduction
The introduction of heterocyclic moieties found in molecules have advantage in drug discovery and development because of its broad range of biological activities. Thiazole and its derivatives show biological activities such as anti-inflammatory,1 analgesic,2 antimicrobial,3,4 antioxidant,5 antitumor,6,7 anticonvulsant.8 3-formylchromone and its derivatives are known to associate in organic synthesis9 and showing biological activities include antitumor,10 antibacterial,10 antitubulin,11 anti-Helicobacter pylori,12 antiallergic,13 antioxidant,14 topoisomerase I inhibitor.15 Pyrazole scaffold heterocyclic molecules are associated with wide range of biological activities such as antimicrobial,16 anticancer,17 antifungal,18 anti-inflammatory,19 antitumor,20 and anti-anxiety.21 Benzoxazoles derivatives are found to be associated with anticancer,22 antimicrobial,23 HIV-1 reverse transcriptase Inhibitor Activity,24 inhibitors of lysophosphatidic acid acyltransferase-beta,25 anti-inflammatory,26 analgesic,26 antibacterial,27 antifungal,27 anticancer28 activities. As a part of our interest in heterocyclic molecules have a extensive variety of biological activities and that have been explored for developing pharmaceutically important molecules, we here in report the synthesis of a set of new series of thiazolyl pyrazoles and benzoxazoles and their antibacterial activities.
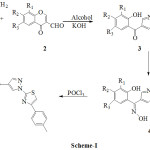 |
Scheme 1 |
Table I: Physical characterisation of synthesised derivatives.
|
Deriva. |
R1 | R2 | R3 | Yield (%) | M.P.(oC) |
| 3a | H | H | Me | 62 | 220-222 |
| 3b | H | H | Cl | 64 | 230-232 |
| 3c | H | Me | H | 60 | 236-238 |
| 3d | H | Me | Cl | 68 | 204-206 |
| 3e | H | H | H | 61 | 186-188 |
| 3f | H | H | Br | 63 | 228-230 |
| 3g | Cl | H | Cl | 65 | 210-212 |
| 4a | H | H | Me | 58 | 232-234 |
| 4b | H | H | Cl | 54 | 246-248 |
| 4c | H | Me | H | 55 | 192-194 |
| 4d | H | Me | Cl | 60 | 250-252 |
| 4e | H | H | H | 52 | 214-216 |
| 4f | H | H | Br | 56 | 206-208 |
| 4g | Cl | H | Cl | 64 | 202-204 |
| 5a | H | H | Me | 67 | 232-234 |
| 5b | H | H | Cl | 54 | 196-198 |
| 5c | H | Me | H | 55 | 202-204 |
| 5d | H | Me | Cl | 53 | 240-242 |
| 5e | H | H | H | 56 | 224-226 |
| 5f | H | H | Br | 52 | 228-230 |
| 5g | Cl | H | Cl | 54 |
244-246 |
Experimental
All the chemicals were from Sigma – Aldrich and used without any purification. Melting Points of synthesised compounds were taken in open capillary tubes and incorrected. IR spectra were obtained in KBr pallet on a FT-IR spectrophotometer and Mass spectra were recorded on a Q- TOF MS ES-3.84e3. 1H NMR spectra were recorded on BRUKER AVANCE II 400 MHz spectrometer with DMSO-d6as a solvent and using TMS as internal standard. Chemical shift (δ) values are expressed in ppm.
(2-hydroxyphenyl)(1-(4-p-tolylthiazol-2-yl)-1H-pyrazol-4-yl)methanone 3
Equimolar mixture of thiazolyl hydrazine 1 (0.01 mol) and 3-formyl chromone 2 (0.01 mol) in ethanol were refluxed for 45 min, hydrazone derivative was obtained. Further, refluxing continues for 4-5 hr by addition of 10 ml 15% potassium hydroxide. After completion of reaction the content was cooled, poured into ice and then neutralised by using c. HCl, solid pyrazolyl methanone derivative 3a produced. Then it was filtered and recrystallised from ethyl alcohol. The compounds 3b-g were synthesised by the same procedure.
3d. IR : 3105, 1654, 1610, 1106 cm-1; Mass: m/z 409.5 (M+); 1H NMR (DMSO-d6): δ 2.37 (s, 6H, Ar-CH3), 6.99 ( s, 1H, Ar-H), 7.24 (d, 2H, Ar-H), 7.60 (s, 1H, Ar-H), 7.81 (s, 1H, Ar-H) 7.87 (d, 2H, Ar-H), 8.23 & 8.96 (s, 2H, Pyrazole), 10.88 (s, 1H, Ar-OH); Elemental analysis- Calculated. C21H16O2N3S Cl: C, 61.53; H, 3.93; N, 10.25. Found: C, 61.55; H, 3.91; N, 10.27 %.
2[(E)–{1-[4-(p-tolyl)-1, 3-thiazol-2-yl)]-1H-pyrazol-4-yl}(hydroxyimino)methyl]phenol 4
To a solution of pyrazolyl methanone 3 (55 mmol) in ethanol, 15 ml 25% potassium hydroxide in water was added at about 0-4oC followed by hydroxylamine hydrochloride (1. 2 mol) in heaps. Once the the addition was complete, continues stirring for 4 hrs at r.t. After reaction completion, content was poured into ice water and neutralised with acetic acid, solid thiozole anchored pyrazolyl oxime 4a separated. It was filtered and recrystallised from ethyl alcohol. The compounds 4b-g were synthesised by same procedure.
4d. IR : 3085, 1612, 1110 cm-1; Mass: m/z 424.5 (M+); 1H NMR (DMSO-d6): δ 2.33 (s, 3H, Ar-CH3), 2.37 (s, 3H, Ar-CH3), 6.89 (s, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.24 (d, 2H, Ar-H), 7.66 (s, 1H, Ar-H), 7.82 (m, 3H, Ar-H), 8.88 (s, 1H, Ar-H), 9.98 (s, 1H, N-OH), 11.88 (s, 1H, Ar-OH); Elemental analysis- Calculated: C21H17O2N4SCl: C, 59.36; H, 4.03; N, 13.19. Found: C, 59.35; H, 4.05; N, 13.17 %.
2-(1-(4-p-tolylthiazol-2-yl)-1H-pyrazole-4-yl)benzo[d]oxazole 5
The thiozolyl oxime 4 (5mmol) in 5 ml POCl3 was taken in round bottom flask and refluxed for about 4 hr. After completion of reaction, cooled content was poured into ice and neutralised with 2% sodium hydroxide, solid separated. Then it was filtered and recrystallised from alcohol to afford oxazole 5a. Compounds 5b-g was synthesised by same method.
5d. IR : 1638, 1241, 1110 cm-1; Mass: m/z 406.5 (M+); 1H NMR (DMSO-d6): δ 2.36 (s, 3H, Ar-CH3), 2.50 (s, 3H, Ar-CH3), 7.30 ( d, 2H, Ar-H), 7.82 (s, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 7.95 (d, 2H, Ar-H), 7.98 (s, 1H, Ar-H), 8.58 & 9.36 (s, 2H, Ar-H); Elemental analysis- Calculated: C21H15ON4SCl: C, 61.99; H, 3.72; N, 13.77. Found: C, 61.97; H, 3.74; N, 13.79 %.
Antibacterial Activity
An antibacterial activity of synthesised compounds 3a-g, 4a-g and 5a-g were determined in vitro against two bacterial species E. coli and B. subtilis. By using agar well diffusion method, bacterial species were cultured on nutrient agar plates at 37oC. Plate containing 20 ml of nutrient agar was spread with 100µl of culture. The wells were made in the agar cork boarer of width of 6 mm. The 100 µl of test compounds were loaded in the well along with amphicilin as positive control and DMSO as vehicle control. The plates incubated at 37oC for 24 hrs. Growth was evaluated visually by comparing a test plate with the control plates. The figures in Table-II indicates inhibition zone in mm and these are the mean of triplicate assays.
Table II: Results of antibacterial activities of synthesised compounds.
| Synthesisedcompounds | Inhibition zone in mm | |
| Bacillus subtilis | Escherichia coli | |
| 3a | 11 | 13 |
| 3b | 9 | 14 |
| 3c | 11 | 15 |
| 3d | 12 | 15 |
| 3e | 15 | 16 |
| 3f | 13 | 10 |
| 3g | 12 | 12 |
| 4a | 13 | 14 |
| 4b | 10 | 15 |
| 4c | 12 | 14 |
| 4d | 11 | 16 |
| 4e | 11 | 16 |
| 4f | 11 | 09 |
| 4g | 13 | 14 |
| 5a | 14 | 11 |
| 5b | 12 | 16 |
| 5c | 13 | 13 |
| 5d | 13 | 14 |
| 5e | 10 | 16 |
| 5f | 12 | 14 |
| 5g | 14 | 09 |
| Amphicilin | 16 | 17 |
Result and Discussion
Synthesised compounds 3a-g were obtained from the thiazole anchored molecule 1 and 3- formyl chromone 2. The yield of 3a-g compounds were in the range of 60-70%.The FTIR spectra of 3d shown peak at 3105 and 1654 cm-1 indicated the presence of Ar-OH and C=O groups in molecule. Where as, the NMR spectra of 3d show the two singlets at δ 2.37 & 10.88 indicated the presence of Ar-CH3 and Ar-OH.The compounds 4a-g were obtained from the compounds 3a-g and hydroxylamine hydrochloride by stirring at room temperature, practical yield of 4a-g compounds were in the range of 55-65%. From the FTIR spectra of 4d, the appearance of peak at 3085 cm-1 indicated the presence of Ar-OH and the disappearance of peak at 1654 cm-1 shown the absence of C=O group. The NMR spectra of 4d shown one singlet at δ 9.98 indicated the presence of –OH group of oxime. The compounds 5a-g were obtained from the refluxing the compounds 4a-g in POCl3, yield in the range of 50-60%. The disappearance of IR peak at 3085 cm-1 shown the absence of Ar-OH in 5d. In the NMR spectra of 5d, absence of two singlet at δ 9.98 and 11.88 of N-OH & Ar-OH, confirmed the formation of compound 5d. Mass spectroscopy also supported for the formation of 3a-g, 4a-g and 5a-g compounds. Physical characterised data of synthesised compounds are given in the Table-I.The antibacterial activities of synthesised compounds are summarised in the Table – II. The figures indicate the zone of inhibition in mm. From the results it is apparent that among synthesised compounds 3d, 4d and 5d have shown good antibacterial activities.
Acknowledgements
The authors are thankful to the P.G. Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar and P.G. Department of Chemistry, New Arts, Commerce and Science College, Parner, Ahmednagar for providing chemicals. Authors are also thankful to the Management of Anekant Education Society, Baramati, Pune for providing necessary research facilities moreover to the Directors, SAIF and CIL, Panjab University, Chandigarh for providing the spectral and analytical data.
References
- Sharma, R. N.; Xavier, F. P.; Vasu, K. K.; Chaturvedi, S. C. & Pancholi, S. S. J. Enz. Inhib. Med. Chem. 2009, 24, 890.
CrossRef - Kalkhambkar, R. J.; Kulkarni, G. M.; Shivkumar, H.; Nagendra Rao, R. Eur. J. Med. Chem. 2007, 42, 1272.
CrossRef - Karegoudar, P.; Karthikeyan, M. S.; Prasad, D. J.; Mahalinga, M.; Holla, B. S. & Kumari, N. S. Eur. J. Med. Chem. 2008, 43, 261.
CrossRef - Mhaske, P. C.; Vadgaonkar, K. S.; Jadhav, R. P. & Bobade, V. D. J. Korean Chem. Soc. 2011, 55(5), 882.
CrossRef - Jaishree, V.; Ramdas, N.; Sachin, J. & Ramesh, B. J. Saudi Chem. Soc. 2012, 16, 371.
CrossRef - Bradshaw, T. D. & Westwell, A. D. Curr. Med. Chem. 2004, 11, 1009.
CrossRef - Hutchinson, I.; Jennings, S. A.; Vishnuvajjala, B. R.; Westwell, A. D. & Stevens, M.F.G. J. Med. Chem. 2002, 45, 744.
CrossRef - Hays, S. J.; Rice, M. J.; Ortwine, D. F.; Johnson, G.; Schwarz, R. D.; Boyd, D. K.; Copeland, L. F.; Vartanian, M. G. & Boxer, P.A. J. Pharm. Sci. 1994, 83, 1425.
CrossRef - Sabitha, G. Aldrichimica Acta. 1996, 29, 15.
CrossRef - Nawrot-Modranka, J.; Nawrot, E. & Graczyk, J. Eur. J. Med. Chem. 2006, 41, 1301.
CrossRef - Wang, B.; Yang, Z-Y. & Li, T. Bioorg. Med. Chem. 2006, 14, 6012.
- Masami, K.; Toru, T.; Hiroyuki, K.; Satoru, T.; Hideki, N. & Hiroshi, S. In. Vivo. 2007, 21, 829
- Fitzmaerice, C. & Wragg, A. H. Chem. Abstr. 1966, 65, 3444.
- Lee, H.; Lee, K.; Jung, J. K.; Cho, J. E. & Theodorakis, E. A. Bioorg. Med. Chem. Lett. 2005, 15, 2745.
CrossRef - Boege, F.; Straub, T.; Kehr, A.; Boesenberg, C.; Christiansen, K,; Andersen, A.; Jakob, F. & Köhrle, J. J. Bio. Chem. 1996, 271, 2262.
CrossRef - Karale, B. K.; Pawar, P. Y.; Gadakh, A. V.; Akolkar, H. N.; & Rindhe, S. S. Indian J. Het. Chem. 2014, 23(3), 283.
- Amandeep, K.; Rashmi, A. & Gill, N. S. Int. J. Nat. Prod. Sci. 2012, 1, 247.
- Zhao, P. L.; Wang, F. & Zhang, M. Z. J. Agreec. Foodchem. 2008, 56, 1076.
- Benard, M.; Hulley, E.; Molenda, H. & Stochla, K. Pharmazie, 1986, 41, 560.
- Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C; Cho, S. Y. & Lee, K. I. Bioorg. Med. Chem. Lett. 2005, 15, 3307.
CrossRef - Wastrow, D. J.; Knobelsdorf, J. A.; Akanne, H.; Mackenzie, D. R.; Pugsley, T. A.; Zoski, K. T.; Heffner, T. G. & Wise, L. D. Bioorg. Med. Chem. Lett. 1998, 8, 2067.
CrossRef - Gadakh, A.V.; Pandit, C.; Rindhe, S. S. & Karale, B. K. Bioorg. Med. Chem. Lett. 2010, 20(18), 5572.
CrossRef - Narwade, S. K.; Karale, B. K.; Jagdhani, S. G.; Chaudhari, C. S. & Rindhe, S.S. Orient. J. Chem. 2008, 24(3), 1029.
- Akbay, A.; Ören, I.; Arpaci, Ö. T.; Sener E. A. & Yalçin, I. Arzneimittel-Forsch. 2003, 53, 266.
- Gong, B. Q.; Hong, F.; Kohm, C.; Bonham, L. & Klein, P. Bioorg. Med. Chem. Lett. 2004, 14, 1455.
CrossRef - Unlu, S.; Baytas, S. N.; Kupeli, E. & Yesilada, E. Arch Pharm. 2003, 336, 310.
CrossRef - Ramalingan, C.; Balasubramanian, S.; Kabilan, S. & Vasudevan, M. Eur. J. Med. Chem. 2004, 39, 527.
CrossRef - Murty, M. S. R.; Ram, K. R.; Rao, R. V.; Yadav, J. S.; Rao, J. V. & Cheriyan, V. T. J. Med. Chem. Res. 2011, 20, 576

This work is licensed under a Creative Commons Attribution 4.0 International License.









