Simultaneous Removal of Arsenite and Fluoride from Groundwater using Batch Electrochemical Coagulation Process - Role of Aluminum with Iron Electrodes
Shruthi Murthy*1 , Mahesh Shivaswamy1
, Mahesh Shivaswamy1 , Sahana Mahesh1
, Sahana Mahesh1 and Srikantha Hanumanthappa2
and Srikantha Hanumanthappa2
1Department of Environmental Engineering, SJCE (JSSS and T University), Mysuru-570006, India.
2Department of Civil Engineering, Alva's Institute of Engineering and Technology, Moodbidri, Mijar, Dakshina Kannada, Karnataka, India.
Corresponding Author E-mail: shruthimvj@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350110
Article Received on : 20-08-2018
Article Accepted on : 14-01-2019
Article Published : 12 Feb 2019
Aluminum(Al) and Iron(Fe) electrodes were used forsimultaneous removal of arsenite and fluoride from groundwater using novel electrochemical coagulation (ECC)with special focus on electrode placing positions of Fe and Al in a batch electrochemical reactor (BECR).A series of experiments were carried out to observe the influence of electrode placing positions on removal. Of the many electrode combination, Al1-Al2-Al3-Al4, Fe1-Fe2-Fe3-Fe4, Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4 are discussed in this paper for pre-optimized operating conditions: 4 plate electrodes, As (III)o: 1.6 mg/L, Fo‾: 12 mg/L, Al3+: 0 mg/L, Feo: 0.061 mg/L, inter-electrode spacing: 5mm, applied cell voltage:16V, SA/V ratio: 40 m2/m3 and electrolysis time of 45 min. For the said electrode combinations,the maximum simultaneous removal of both arsenite and fluoride was obtained for Fe1-Fe2-Al3-Fe4 with 97% arsenite removal from its initial value of 1.6 mg/L; and 100% fluoride removal from its initial value of 12 mg/L within 45 min of ET. Energy consumption was 2.01 KWh/m3 with operating cost of 2.90 Rs./m3.
KEYWORDS:Aluminum; Arsenite Andfluoride Removal; Electrode Placing Position; Iron Electrodes
Download this article as:| Copy the following to cite this article: Murthy S, Shivaswamy M, Mahesh S Hanumanthappa S. Simultaneous Removal of Arsenite and Fluoride from Groundwater using Batch Electrochemical Coagulation Process - Role of Aluminum with Iron Electrodes. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Murthy S, Shivaswamy M, Mahesh S Hanumanthappa S. Simultaneous Removal of Arsenite and Fluoride from Groundwater using Batch Electrochemical Coagulation Process - Role of Aluminum with Iron Electrodes. Orient J Chem 2019;35(1). Available from: https://bit.ly/2UUmMdk |
Introduction
Providing clean and potable water to people is a challenging task. Fresh water contamination is a worldwide health related issue requiring great attention because of its hazardous effects, risks to human health and economic damages1 as well. Among the wide variety of contaminants affecting fresh water resources, arsenic and fluoride are important water quality parameters because of its extreme toxicity potential even at low concentrations. Long-term exposure to inorganic arsenic causes many adverse human health effects including cardiovascular, hepatic, and renal diseases in addition to cancer in kidney, liver, lungs, urinary bladder, and skin. Arsenic in drinking water is also linked to lung cancer and other diseases including peripheral neuropathy, skin lesions,skin cancer and bladder cancer.2 Table 1 shows the health effects from arsenic concentrations in water.
Table 1: Arsenic health effects.
| Health Effects | Concentration in water |
| IQ deficit | 10 µg/L, children |
| 11 µg/L, children; 30 µg/L , adults | |
| Skin Keratosis | 50 µg/L |
| Artery, arteriole and capillary disease | 20-91.5 µg/L |
| Cerebral infarct | 166 µg/L |
| Cerebrovascular disease | 189 µg/L |
| Abnormal electromyograms | 60-140 µg/L |
| Spontaneous abortion and perinatal death | 60-470 µg/L |
Similarly, the detrimental effects of long-term ingestion or exposure to high concentrations of fluoride in drinking water are known for physiological disorders, dental and skeletal fluorosis, thyroxine changes, and kidney damage.3 Table 2 shows different human health effects ranging from <0.5 to >10 of fluoride in drinking water. The World Health Organization (WHO) sets the maximum contamination level (MCL) for arsenic as 10µg/L. Similarly, the maximum acceptable concentration of fluoride anions in drinking water set by WHO is 1.5 mg/L at rejection level4 and lower values at acceptable levels of 1.0 mg/L; the same values are retained in 5(BIS-Bureau of Indian Standards : 10500-91, 2003. India).
Table 2: Health Effects of Fluoride.
|
Fluoride value in water, mg/L |
Health effects |
| <0.5 | Dental caries |
| 0.5-1.5 | Promotes dental health |
| 1.5-2.5 | Dental fluorosis |
| 2.5-4 | Dental fluorosis |
| >4 | Dental, skeletal fluorosis |
| >10 | Osteosclerosis |
The increased attention to arsenic and fluoride toxicity on human beings has incited considerable research for developing new robust and reliable methods for removing arsenite and fluoride from groundwater. Past treatment processes for arsenic removal were coagulation/filtration,6 manganese green sand filtration, reverse osmosis, electro dialysis reversal and oxidation/filtration, ion exchange, adsorption using adsorbents such as activated carbon, granular ferric oxides, iron oxide coated sand.7,8 The most popular processes for drinking water defluoridation are adsorption using activated alumina9 bone char,10 activated carbon11 and coagulation using aluminum salts.12 Other defluoridation processes include electrodialysis,13 reverse osmosis14 and nano filtration.15 These processes show high HRT, expensive set-up, high capital costs, not economically viable for small communities and alsonotable to treat concentration surges in arsenic and fluoride values and also very high maintenance requirements. Moreover, the above treatment methods require a long treatment train with number of unit operations and unit processes with pH adjustments as well as addition of non-stoichiometric acid and alum coagulants, ferric sulphate/chloride, lime, caustic/polymeric flocculants and follow-up retrofits.Furthermore, these processes defectively generate secondary pollutants like chlorides and sulphate in the coagulation-precipitation process, loaded with unreacted chemicals and large volumes of sludge demanding further treatment and safe disposal of solid residues. In view of the above issues, research intensification for developing an alternate novel treatment processes for simultaneous removal ofarsenic and fluoride at low HRT,minimum operation and maintenance costis much needed.
ECC as a novel treatment technology utilizes less current for the dissolution of metal electrodes and higher treatment. Depending on the electrode selected the dissolved M+ ions at wide range of pH forms coagulating species and metals hydroxides. These hydroxides have potential to destabilize even the smallest negatively charged particles which precipitate and adsorb the dissolved contaminants. Finally, precipitate removal by electro-flotation or by sedimentation by bubble-buoy-adhesion and transport to the top of the bulk solution for maturation and aggregation, where the sludge get sseparated later down line.
Previous researchers have mainly focused on ECC treatment efficiency for either arsenic or fluoride removal in separate studies. In contrast, we investigate to assess the best positions of iron and aluminum electrodes inside the ECC reactor in bipolar arrangement for simultaneous removal of fluoride and arsenic from groundwater. The appropriate electrolysis time (ET) required for each combination is based on the stable/matured state of the flocs matrix in the reactor and target levels of fluoride and arsenic, energy consumption, electrode dissolution, sludge generation, passivation factor, quality of the ECC treated water and above all the operating cost.
Materials and methods
Chemicals and Analytical methods
All the chemicals used in this study were of analytical reagent (AR) grade obtained from Hi-Media laboratories Pvt. Ltd, Mumbai, India. The analysis of various physico-chemical water quality parameters were carried out using various equipments and instruments. pH was measured using pH meter (LI 127 Elico make), electrical conductivity using a digital conductivity meter (Systronics model 30/10 FT YSI) and temperature variations recorded using a digital thermometer. Iron, nitrate, fluoride, sulphate and phosphate concentrations were determined using UV-spectrophotometer. Total hardness, total alkalinity and TDS was determined as per Standard Methods16 (APHA, 2017). Aluminum and arsenic concentrations in the solution were determined using Inductively Coupled Plasma (ICP Horiba Jobin Yvon, France).
Characterization of Groundwater
Groundwater samples was collected as and when required from a nearby groundwater source and analyzed for various drinking water quality parameters following the Standard methods (APHA, 2017) and is presented in Table 3.These parameters were analyzed to have clear idea about the chemistry of the water undergoing the ECC process for complete understanding of the interactions that occurin the ECC reactor. Arsenite stock solution of 1000 mg/L was prepared by dissolving desired amount of sodium meta arsenite to spike the sampled ground water to obtain the desired initial concentration) before ECC. Similarly, fluoride stock solution was prepared to get the desired concentration as for experimental use. Arsenite was chosen for ECC treatment because it is 25-60 times more toxic and mobile, in groundwater aquifers whereas, fluoride causes irreversible and health issues. Arsenite and fluoride removal is been a challenging task in ground reality to make it potable.
Table 3: Initial Characterization of Groundwater before Electrochemical treatment.
|
Sl. No. |
Water quality parameter |
Units |
Characterized values |
BIS (IS 10500-91, Revised 2003) |
|
|
Desirable limit |
Permissible limit |
||||
| 1 | Color | Hazen | Colorless | 5.0 | 25 |
| 2 | Temperature | °C | 22-29 | – | – |
| 3 | pH |
– |
7.52-7.73 | 6.5-8.5 | No relaxation |
| 4 | Total Alkalinity as CaCO3 | mg/L | 360-377 | 200 | 600 |
| 5 | Electrical Conductivity | µS/cm | 807-1075 | – | – |
| 6 | Turbidity | NTU | 0.29-1 | 5 | 10 |
| 7 | Chloride | mg/L | 70-91 | 250 | 1000 |
| 8 | Total Hardness as CaCO3 | mg/L | 352-463 | 300 | 600 |
| 9 | Calcium Hardness as CaCO3 | mg/L | 140-160 | 75 | 200 |
| 10 | Magnesium Hardness as CaCO3 | mg/L | 212-253 | 30 | 100 |
| 11 | Iron | mg/L | 0.01-0.03 | 0.3 | 1.0 |
| 12 | Fluoride | mg/L | 1.08- 1.10 | 1.0 | 1.5 |
| 13 | Arsenic | mg/L | Nil | 0.01 | 0.05 |
| 14 | Aluminum | mg/L | Nil | 0.03 | 0.2 |
Experimental Setup
The laboratory scale batch electrochemical coagulation (BECC) unit was designed and fabricated using organic glass to function as electrochemical reactor (ECR). The experimental set up comprised of an ECC reactor of cubical shape having an effective volume of 2L with internal dimensions 12 cm x 11cm x 17cm. The electrode holding arrangements were made of perspex glasson the inner opposite sides of the walls of the reactor. A larger depth for inductive stirring at the bottom of the vertical electrode plates was provided for achieving effective mixing of the bulk fluid in the ECR. Mixing of bulk solution in ECR was achieved by means of an inductive magnetic stirrer (REMI 2MLH) with rotation speeds optimized at 410 rpm. Iron and aluminum sheets of dimensions 10cm x 10 cm x 0.1 cm were arranged bipolar in parallel keeping inter-electrode distance of 5mm. The current input from DC power supply unit was maintained constant using a precision DC power supply (Textronix 35D, Dual regulated powers supply, 0-16V, 0-10A) unit. The DC power supply unit was switched on with apre-optimized cell voltage kept at 16V which was obtained as an optimum voltage in preliminary studies.All the batch ECC runs were performed at room temperature.
In each experimental run, 2L of arsenite and fluoride spiked ground water was fed into the reactor. All the BECC experiments were carried out for various electrode placing positions in the ECR (Fig.1(a) and 1(c)). Samples were retrieved at regular time intervals, filtered and analyzed for residual arsenite, fluoride, iron and aluminum concentrations after each ECC run. After ET (electrolysis time), the electrodes were taken out of the ECR, a gentle mixing was attained for treated water using a glass rod so that the flocs again re-flocculateto form larger floc matrix and settle down in the reactor in <5 min is shown in Fig. 1(b) and 1(d). Later, the supernatant was decanted and filtered for further analysis for the water quality parameters. The wet solid residue/sludge was collected in porcelain dishes, dried in a hot air oven at 110°C. The dried sludge was further subjected to physico-chemical and elemental characterization. At the start and end of each experimental run, the electrodes were washed thoroughly with water followed by 15% HCl solution to release entrapped flocs in pits of the electrode faces and again washed with distilled water, dried and weighed. The difference in weight was noted for each electrodes because this information was used to estimate electrode consumption.
 |
Figure 1: Experimental set up of Laboratory scale batch electrochemical coagulation unit |
(a) ECR with aluminum electrodes for As (III) & F− removal (b) Aluminum hydroxide sludge settled using Al1-Al2-Al3-Al4 at the bottom of the beaker after ECC (c) ECR with iron & aluminum electrodes for As (III) & F− removal (d) Fe(OH)3 sludge using iron and aluminum electrodes.
Results and Discussion
In any electrochemical treatment process, different electrode materials and compatible electrode combinations are regarded as significant factors influencing the performance of the ECC process.17 The appropriate selection of the electrode material and its position in the ECR play an important role in delivering the proper M+ ions to remove fluoride and arsenite simultaneously from water/ground water.To study the effectof type of electrode (Al and Fe) and its positions in the reactor for simultaneous removal of arsenite and fluoride from groundwater,a series of 16 sets of experiments were carried out for different electrode bipolar combinations like: (1) Al1-Al2-Al3-Al4,(2) Al1-Fe2– Fe3-Fe4(3) Al1-Fe2-Fe3-Al4(4) Al1-Al2-Fe3-Al4(5) Al1-Al2-Al3-Fe4 (6) Al1-Fe2– Al3-Fe4 (7) Al1-Al2-Fe3-Fe4(8) Al1-Fe2-Al3-Al4 (9) Fe1-Fe2-Fe3-Fe4(10) Fe1-Fe2-Al3-Al4(11) Fe1-Al2-Al3-Al4 (12) Fe1-Al2-Al3-Fe4 (13) Fe1-Fe2-Fe3-Al(14) Fe1-Fe2-Al3-Fe4(15) Fe1-Al2-Fe3-Fe4 and (16) Fe1-Al2-Fe3-Al4.
Out of the above said 16 sets of bipolar electrode arrangements, the results of only four sets of experiments are presented for discussion because other electrode arrangements showed poor As and F− removal and also other removal of water quality parameters. The four electrode arrangements discussed are: (1) 4 aluminum electrodes (Al1-Al2-Al3-Al4), (2) 4 iron electrodes (Fe1-Fe2-Fe3-Fe4) (3) 3 Aluminum electrodes and 1 iron electrode (Al1-Al2-Fe3-Al4), and (4) 3 iron electrodes and 1 aluminum electrode (Fe1-Fe2-Al3-Fe4). Fig. 2 (a-d) shows these four different electrode configurations in bipolar arrangement with electrodes placed in parallel.
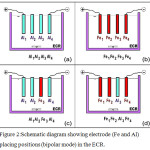 |
Figure 2: Schematic diagram showing electrode (Fe and Al) placing positions (bipolar mode) in the ECR. |
Effect of Using 4 Aluminum (4 Al) Electrodes for Simultaneous Removal of Arsenite and Fluoride
Batch ECC experiments were carried out using four Al plate electrodes arranged in parallel having SA/Vof 40 m2/m3. With a bipolar connection, the electrode arrangement was: Al1-Al2-Al3-Al4; at an inter-electrode spacing of 5 mm operated at a cell voltage of 16V and a corresponding current of 0.61 A for 45 min ET with background Fe and Al concentrations in groundwater was 0.061 mg/L and 0 mg/L respectively with initial pHo of 8.9.
 |
Figure 3: (a) Variations in fluoride and arsenite concentrations during batch ECC using 4 aluminum electrodes. |
(b) Variations in fluoride and arsenite concentrations during batch ECC using 4 iron electrodes. (c) Variations in fluoride and arsenite concentrations during Batch ECC using 3 Al+1 Fe Electrodes. (d) Variations in fluoride and arsenite concentrations during batch ECC using 3 Fe+1 Al Electrodes.
Fig. 3(a) shows the reduction in fluoride and arsenite concentration with time during the ECC process. Fluoride concentrations reduce significantly compared to arsenite in the bulk solution.Fluoride concentrations reduced to<0.3 mg/L from its Co value of 12 mg/L. During the first 5 min of ET, Al3+ ions released from the anode making the bulk solution turbid with 9.86 mg/L of fluoride remaining in solution;at this time Alconcentration in the bulk solution was 0.017 mg/L proving the liberation of Al3+ as charged in-situ coagulants required for floc formation in the presence of sufficient alkalinity ranging from 360-377 mg/L. During this time, a slight increase in the bulk solution temperature occurs. As a result of exothermic reactions, the hydrated trivalent aluminum ion undergoes hydrolysis generating various monomeric, dimeric, trimeric and polynuclear hydrolysis products as shown through equations (1)-(3) by.18
Al3+ + H2O → Al(OH)2+ + H+ (1)
Al(OH)2+ + H+ → Al(OH)2++ H+ (2)
Al(OH)2++ H2O → Al(OH) 3+ H+ (3)
These H+ ions make water near the anode slightly acidic and because of continuous inductive stirring, slowly the pH of the bulk solution drops down from 8.9 to 7.8 at 30 min ET with a fluoride reduction of 90% F−o: 12 mg/L, F−30: 1.26 mg/) following the reaction shown in equation (4).
Al(OH)3+ xF− → Al(OH)3—x Fx + xOH– (4)
The fluoride ions exchanges partially with OH– ions in the Al(OH)3 matrix to free the OH– causing a slight increase in pH from 7.8 to 8.1 at 45th min of ET. Arsenite showed a meager 55% removal at 45min ET from its initial concentration of 1.6mg/L; this level of removal is assumed to have occurred because of the background iron concentration in the bulk solution. Al3+ ions showed unsatisfactory results on arsenite removal compared to the removal of fluoride. The moment fluoride values reduced to 1.26 mg/L at 30th min ET, the corresponding aluminum concentration increased to 0.3 mg/L at 45 min ET exceeding the desirable BIS drinking water standards of 0.03 mg/L indicating the presence of soluble Al(OH)4– formed at ˜ pH 8.Contrastingly, iron concentration remained constant (dashed line in Fig. 3a) throughout the ET showing no evidence of iron in the bulk solution.
After the completion of electrolysis, the temperature of the bulk solution To :22°C) showed an increase by 3.5°C because of various redox and displacement reactions that occur within the ECR. The flocs formed during the ECC process is a jel like matrix.It was concluded that use of 4aluminumelectrodes was effective in fluoride removal with a marginal contribution to the removal of arsenite.
Effect of using 4iron electrodes (4 Fe)
In another set of experiments, batch ECCwas carried out using 4 Fe electrodes with40 m2/m3 SA/V.Fe1-Fe2-Fe3-Fe4 represents four iron electrode positions in the ECR with Fe1 connected to the positive terminal and Fe4 connected to the negative terminal of the DC power supply unit (Fig. 2b). The other operating parameters andinitial values remained unchanged except for the backgroundiron and aluminum concentration of 0.061 mg/L and 0 mg/L respectively.
From the plot (Fig. 3(b)), itis observed that a very small decrease in the fluoride removal from 12 mg/L to 11.83 mg/L is observed, while arsenite concentration decreased from 1.6 mg/L to 0.081 mg/Lat 45 min ET. During the process, the physical color of water changed from colorless to rust yellow within first 5 min because of the in-situ generation of Fe2+ metal ions at the face of anode, beginning with the reactions shown in equations (5) and (6):
Fe → Fe2+ + 2e– (5)
Fe2+ + 2e– → Fe3+ + 3e– (6)
Continuing with the reactions in equation (5) and (6), the bulk solution color turns pale yellow ascribed to the formation of Fe(OH)2; these species are soluble and therefore floc formation in the bulk solution wasn’t possible. As the ECC treatment progressed, the pH of the solution changed from acidic to alkaline resulting in color change of the bulk solution from yellow to green and then to blue. Ferric ions reacts with OH– ions forming ferric hydroxide by the reaction (7).
Fe3+ + 3OH– → Fe (OH)3 (7)
Formation of Fe(OH)3 leads to red brown colloidal solution. Arsenate co-precipitates with Fe(OH)3 and thus, arsenate gets removed from the bulk solution and the equation (8) justifies this statement:
Fe(OH)3 + AsO43– → Fe(OH)3* AsO43– (8)
As a result of the reaction in equation (8), the pH of the bulk solution increased from 8.83 to 9.45. No changes in the bulk solution temperature were observed during the first 15 min after which the temperature increased by 1°C at 45 min ET; it is inferred that while using iron electrodes, the reaction occurring in the cell is less exothermic than when using aluminum electrodes. The residual iron concentration in the treated water at 45 min ET was 0.066 mg/L which was well within the prescribed drinking water quality standards as per BIS – 10500 1993 of India and WHO.
Effect of using three aluminumelectrodes and one ironelectrode (3 Al and 1Fe)
Batch ECC experiments was carried out by placing aluminum plates in 1st, 2nd and 4th position and iron electrode placed in the 3rd position in the ECR (Fig. 2c). Al1-Al2-Fe3-Al4 represents 3 Al electrodes and 1Fe electrode. Al1 is connected to the positive terminal, Fe3 is placed in the 3rd position and Al4 is connected to the negative terminal of the DC power supply unit; other operating conditions being the same. The background Fe and Al concentration before ECC was 0.061 mg/L and 0 mg/L respectively.
From Fig. 3(c) simultaneous improvements in the reduction of both F– and As (III) was observed at 45 min ET compared to the previous two electrode arrangements. During the treatment process, the pH of the bulk solution reduced from 7.79 to 7.58 with a noticeable color change from colorless to pale yellow within 5 min of ET because of Fe3+ ions and then again changed to green color by 15 min ET because of Fe2+ and Fe(OH)2 ions. The quantity of Al ions Al3+ generated in the beginning of electrolysis is limited and not sufficient enough to precipitate all F– from water; the removal of As(III) ions was comparatively quicker; however, the concentration of fluoride and arsenite at 45th min ET was above the stipulated standards.
The residual Al and Fe concentration in solution were also measured during ECC, it was observed that Fe electrode dissolution was more quickly than the Al electrodes which provides cross evidence for an increase in Fe concentration from 0.074 mg/L to 0.451 mg/L in the bulk solution. At 45th min ET, the residual Al concentration in water was 0 mg/L where as the Fe concentration was 0.45 mg/L little over the limit of 0.3 mg/L. This situation probably demands an additional ETto reach a matured state, where Fe concentration from the solution slowly migrate into the floc matrix and finally into the dry sludge keeping the water free of metal residues.
Effect of Using Three Ironelectrodes and one Aluminum Electrode (3Fe and 1Al)
Iron electrodes were placed parallel in the 1st, 2nd and 4th position while one aluminum electrode placed in the 3rd position in the ECR in bipolar arrangement; with other operating conditions remaining the same (see sub-caption of Fig: 4.2(d)). The background Fe and the Al concentration before ECC was 0.061 mg/L and 0 mg/L respectively. Fe electrode in position 1 behaves as an anode while Fe electrode in position 2 behaves both as an anode as well as cathode showing bipolar behavior. Similarly, the Al electrode in position 3 behaves both as a cathode as well as anode, while the iron electrode in the 4th place (Fe1-Fe2-Al3-Fe4) behaves only as a cathode as seen in Fig. 2(d). Electrodes in positions 2 and 3 show hybrid behavior for releasing the respective metal ions into the bulk solution. In the first 5 min of electrolysis, the bulk solution color changes from colorless to rust brown – light green and then to turbid white. The green and yellow mixed color is ascribed to the release of Fe2+ and Fe3+ ions generated during the EC process by anodic oxidation. Fe2+ is the common ion generated in-situ with relatively high solubility in acidic or neutral conditions which easily gets oxidized into Fe3+ using dissolved oxygen in water. The change in color occurs from colorless to rust brown at 5 min ET (pH: 7.99-7.70); rust brown to light green between 5 and 15 min with pH: 7.70 – 7.74 and finally the solution turns turbid white from light green between 15 and 45 min (pH: 7.76 – 7.78). This sequential change in color ofthe bulksolution at the end of 45 min is accompanied with a small decrease in the pH value of the bulk solution as reasoned out by the reactions illustrated in the equations 9-11. These reactions simultaneously occurin the vicinity of the active face of the aluminum electrode making the solution turbid white as an evidence of Al(OH)2 floc formation and also the formation of FeCl2. The decrease in pH is because of the liberation of H+ ions into the bulk solution.
Al3+ +H2O → Al(OH)2+ + H+ (9)
Al(OH)2+ + H2O → Al(OH)+2 + H+ (10)
Al(OH)+2 + H2O → Al(OH)3 + H+ (11)
It may be observed from Fig. 3(d), as the operating time increases from 0 to 15 min, arsenite and fluoride concentration reduces from 1.6 to 0.072 mg/L and 12 to 2.54 mg/L respectively. It was noticed at 45 min ET, the concentration of arsenite was 0.042 mg/L and fluoride value of 0 mg/L.
The retrieved samples were also analyzed for any left overs of residual Al and Fein the solution. The liberation of Fe ions increased from 0.073mg/L to 0.279 mg/L from 0 – 10 min ET and later the concentration of Feions in the bulk solution showed a constant value of 0.1 mg/Lup to 45 mins, with values well within the BIS drinking water quality standards. With only oneAl electrode in the 3rd position, Al dissolution was meagre and therefore the concentration in the solution was 0 mg/Lin all the sample withdraw alanalyzed reflecting the complete utilization of liberated active aluminum ions in the formation of aluminum hydroxides. The liberated Al ions quickly precipitatein floc formation causing fluoride removal.
Conceptually, a complete EC treatment involves three stages-destabilization, aggregation and maturation. The first stage is usually very short- few seconds to minutes involving charge neutralization,the second stage is relatively long which take more time. The last stage takes few minutes to mature where ‘sweeps flocculation’ occurs. The Fe1-Fe2-Al3-Fe4 electrode combination showed nearly 100% fluoride removal with decrease in F−concentration from 12 mg/L to 0 mg/L with a multitude reduction in As(III) concentration reaching a value of 0.04 mg/L in 30-45 min from its initial concentrations of 1.6 mg/L. The residual Al and Fe concentration in the treated water were < 0.03 mg/L and 0.3 mg/L respectively by the end of 45 min of electrolysis which is well within the stipulated BIS and WHO standards for drinking water, when compared with other electrodes combinations described earlier. An improved performance was observed while using both Al and Fe electrodes in a single reactor because of the simultaneous formation of both Al and Fe hydroxides which complement each other in removing both fluoride and arsenite from water. Al dissolution tends to reduce the pH of the water, while iron increases the pH of water. The combined effect is that both arsenite and fluoride get removed as explained in the reactions (12) and (13).
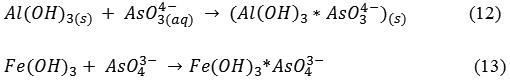
The Al(OH)2 flocs formed during electrolysis binds the arsenate present in the water by the mechanism of charged adsorption and the arsenite electro-removal process is by ferric flocs produced by the. Of all the electrode combinations in different positions in the ECR,the Fe1-Fe2-Al3-Fe4 combination proved an ideal electrode arrangement in the reactor to achieve simultaneous removal of F– and As(III) from ground water.
Effect of Al and Feelectrode Placing Positions Onwater Quality and Production of Secondary Contaminants in Treated Water After ECT
ECC after 45 min ET for all the electrode combinations, the treated water was checked for the presence of secondary contaminants and compared with the BIS-10500 for drinking water. The water quality parameters analyzed for various combinations are shown in Table 4 for various physico-chemical parameters before and after the ECC to observe the changes in the treated water quality.
Table 4: Water constituents before and after the ECC treatment process.
|
Sl. No. |
Water Quality Parameter |
Unit |
BIS-10500, 1993 (Desirable limit) |
Parameter value before ECC |
After 45 min of ECC for operating conditions: 16 V; As(III)0: 1.6 mg/L; F–o : 12 mg/L |
|||
|
Al1-Al2-Al3-Al4 |
Fe1-Fe2-Fe3-Fe4 |
Al1-Al2-Fe3-Al4 |
Fe1-Fe2-Al3-Fe4 |
|||||
| 1 | pH | – | 6.5-8.5 | 7.52-7.73 | 8.1 | 9.45 | 7.58 | 7.76 |
| 2 | Conductivity | µS/cm | 300 | 807-1075 | 637 | 703 | 644 | 810 |
| 3 | Turbidity | NTU | 5 | 0.29-1 | 1.2 | 1.4 | 4.5 | 1.2 |
| 4 | Total Alkalinity as CaCO3 | mg/L | 300 | 360-377 | 176 | 284 | 204 | 240 |
| 5 | Chloride | mg/L | 250 | 70-91 | 72 | 76 | 72 | 72 |
| 6 | Total Hardness as CaCO3 | mg/L | 300 | 352-463 | 148 | 216 | 140 | 176 |
| 7 | Calcium | mg/L | 75 | 140-160 | 72 | 96 | 88 | 88 |
| 8 | Magnesium | mg/L | 30 | 212-253 | 76 | 120 | 52 | 88 |
| 9 | Iron | mg/L | 0.3 | 0.01-0.03 | 0.061 | 0.066 | 0.451 | 0.17 |
| 10 | Aluminum | mg/L | 0.03 | 0 | 0.392 | 0 | 0 | 0 |
| 11 | Fluoride | mg/L | 1-1.5 | 12 | 0.75 | 11.83 | 4.12 | 0 |
| 12 | Arsenic | mg/L | 0.01 | 1.6 | 0 | 0.872 | 0.092 | 0.042 |
pH of the ECC treated water for all the four different electrode combinations increased from its initial pH as a result of OH– at the cathode face,because of the buffering character of Al(III) species especially in this pH region. From Table 4, it may be seen that for Al1-Al2-Al3-Al4, Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4 at the end of 45 min ET, the pH value reached 8.1, 7.58 and 7.76; the values were well within the BIS-10500 desirable limit. When using all 4 iron electrodes(Fe1-Fe2-Fe3-Fe4), the pH of treated water was alkaline (pH: 9.45) exceeding the pH limit of 6.5 – 8.5 with an obvious inference that iron electrodes generate more OH– ions in the presence of sufficient alkalinity. The conductivity of the ECC treated water decreased from its initial value ascribed to the removal of various anions and cations by various redox and chemical coagulation reactions that occur during the electrolysis process. A major portion of the conductivity value is utilized in floc formation. The decrease in conductivity indicates ion pairing or multiple-ion association between solvated species and opposite charges.19 It may be observed from Table 4, conductivity value after treatment for Al1-Al2-Al3-Al4, Fe1-Fe2-Fe3-Fe4,Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4 were 637, 703, 644 and 810 µS/cm respectively from its initial values of 807-1075 µS/cm. The turbidity values after ECC with Al1-Al2-Al3-Al4, Fe1-Fe2-Fe3-Fe4,Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4 electrode combinations were 1.2, 1.4, 4.5 and 1.2 NTU respectively which is greater than the initial values.
The increase in turbidity is because of micro gas bubbles which quickly buoy up along with the metal ions from the solution. Sometimes, the dissolved Al gets polymerized as Al(OH)2 thereby increasing the turbidity of water.
In the post ECC supernatant, the total alkalinity (as CaCO3) value decreased by 21.11-53.31% for all the electrode combinations to safe levels from its initial concentration of 360-377 mg/L. The decrease in total alkalinity after ECC is because of the consumption of alkalinity salts such as carbonates and bicarbonates during the ECC process. The reactions in equations (14), (15) and (16) releases CO2 gas leading to slight increase in the pH of the bulk solution leading to alkalinity consumption.
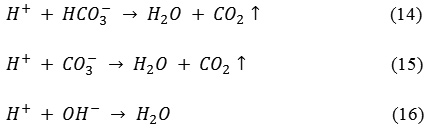
Also, Mg ions present in the bulk solution reacts with carbonate ions forming magnesium carbonate Equation 17) and hence for the both magnesium ions and alkalinity reduce with ET.
![]()
In simple terms, a sufficient background alkalinity in the water become very useful in floc formation; else, in its absence, the water becomes turbid and toxic with large releases of M+ and non – participation of these M+ ions not picking up the negatively charged contaminants/ pollutants.
Presence of sufficient useful salts (Cl–, Na, Ca and Mg) in water reduces the treatment time and promotes floc formation in the presence of total alkalinity. Chloride values decreased by an average of 8.75 % for all the electrode combinations after ECC treatment. As reported by20 the oxidation process converts, chloride ions into chlorine gas causing a reduction in chloride concentration in the ECC treated supernatant. The chlorine gas so released is used as an oxidant to convert ferrous iron to insoluble ferric iron. The presence of ferrous ions and chloride ion in the effluent may alsotend to form amorphous Fe(OH)3 as reported by21 and as a result, the concentration of the chloride ions is reduced from its initial concentration after ECC by the reactions (18), (19) and (20).
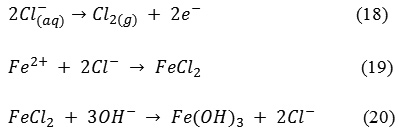
The total hardness value after ECC for Al1-Al2-Al3-Al4, Fe1-Fe2-Fe3-Fe4,Al1-Al2-Fe3-Al4and Fe1-Fe2-Al3-Fe4 electrode combinations were 148, 216, 140 and 176 mg/L respectively from its initial value of 352-463 mg/L. Precipitation of the calcium and magnesium ions to Ca(OH)2 and Mg(OH)2 into the gel matrix results in the hardness removal as shown in the equations (21) and (22).
Ca2+ + OH–→ Ca(OH)2 (21)
Mg2+ + OH–→ Mg(OH)2 (22)
Calcium and magnesium ions plays a very important role in adsorption and precipitation of ions as shown by the reactions in (23) and (24). These reactions occur near the cathode face of the respective electrode when the water pH crosses 8. The added benefit is that contamination in hard water (ground water) is easy to remove compared with waters/wastewater that are soft. In addition to the removal of fluoride and arsenic, the water becomes softer after ECC because Ca & Mg ions get removed from the solution.
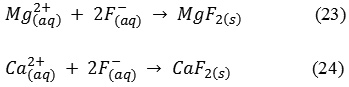
For any drinking water treatment process, using Al and Fe salts,the residual Al and Fe ion concentration in water is an important consideration. The electro chemically treated water samples were checked for the presence of aluminum and ferrous/ferric ions. It was observed that Al and ferrous/ferric ions concentration in solution changed for each combination of the electrodes. It maybe observed in Table 4 for Al1-Al2-Al3-Al4 electrode combination, the residual Al is 0.392 mg/L which is against the stipulated BIS – 10500 standard of 0.03 mg/L& WHO standard of 0.2 mg/L, whereas, the residual Al concentration was 0 mg/L for Fe1-Fe2-Fe3-Fe4, Al1-Al2-Fe3-Al4and Fe1-Fe2-Al3-Fe4 electrode combinations. Residual Fe concentrations for Al1-Al2-Al3-Al4,Fe1-Fe2-Fe3-Fe4 and Fe1-Fe2-Al3-Fe4 were 0.061, 0.066 and 0.17 mg/L respectively, well within the BIS – 10500 and WHO standards,but for Al1-Al2-Fe3-Al4 the residual Fe concentration was 0.451 mg/L, marginally higher than the permissible value of 0.3 mg/L. Therefore, by considering both water quality and simultaneous arsenite and fluoride removal,the Fe1-Fe2-Al3-Fe4 electrode combination was seen best suitable for pollutant removal.
An important outcome is that when using both Fe and Al electrode combinations, the ECC treatment does not impart any significant Fe and Al ion residues into water marking the useful potential of ECC without any sort of secondary contamination of treated water.
Effect of electrode type and position in the ECR for electrode dissolution (ED),energy consumption, sludge quantity and operating cost (OC)
Major issues for accepting the use of ECC is the electrode consumption (ED), post ECC water quality, sludge generation, operating cost (OC) and energy consumption. Fig. 4(a-e)illustrates the effect of different electrode positions on ET for the removal of excess arsenite and fluoride to meet the prescribed drinking water quality standards. As seen in Fig. 4(a), the Fe1-Fe2-Al3-Fe4 electrode placing position showed electrode dissolution 0.667 Kg/m3 in 45 min ET. It may be observed that with more iron electrodes than Ali.e. for Fe1-Fe2-Fe3– Fe4 and Fe1-Fe2-Al3-Fe4 electrode combinations, the electrode dissolution from the Fe electrode is 0.606 and 0.498 Kg/m3. While using only Al1-Al2-Al3-Al4 and Al1-Al2-Fe3-Al4 combinations, the amount of Alions liberated is significantly less: 0.303 and 0.214 Kg/m3.
The gross sludge quantity generated for the four different electrode combinations were 0.955, 0.99, 0.915 and 1.115 Kg/m3 for Al1-Al2-Al3-Al4;Fe1-Fe2-Fe3-Fe4; Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4 combination respectively and the same is plotted in Fig. 4(b). The quantity of sludge generation reflects floc formation by active electro-coagulating agents; using the Fe1-Fe2-Al3-Fe4 combination both Fe(OH)2 and Al(OH)3 formation occurs as reported by22 explained by the equation (25).
Fe(s) + Al(s) + 2H2O = Fe(OH)2 +Al (OH)3 +H2(g) (25)
Operating Cost (OC)
The success of any water/ wastewater treatment option is its ‘Operating cost’. In the ECC treatment process, the OC includes material electrodes cost, utility cost (electrical energy), labor, maintenance and other fixed costs.23 The major components like – cost of energy, electrode material and chemicals (Equation 26) for the treated water were taken into account to arrive at the ‘operating cost’ in Indian Rupee (INR) for each cubic meter of water treated.
Operating Cast = a Cenergy + b Celectrode +c Cchemical (26)
where, Cenergy is the energy consumption in kWh per m3 of water treated using equation (27).
![]()
Celectrode is the electrode consumption in kg per m3 of water treated that is calculated as shown in equation (28).
![]()
where, I is the current (A), t is the electrolysis time in seconds, M is the molecular mass of iron (0.05585 kg/mol) and aluminum (0.02968 k/mol), Z is the number of electrons transferred for iron (ZFe = 2) and aluminum (ZAl = 3) F is the Faraday’s constant (96485 C/mol) and V is the volume of effluent treated in m3. Cchemicals is the chemical consumption in kg per m3 of water treated. The unit prices a, b and c for the Indian market are as follows: ‘a’is the electrical energy prices of 0.0065 US $/ KWh; ‘b’ is electrode material price as 0.3 US $/kg averaged for aluminum and iron respectively, ‘c’ is price of chemicals which is zero Rs./kg, as no chemical are used as additives in the treatment process.
The OC for different electrode combinations were calculated using equation (26) for the simultaneous removal of arsenic and fluoride from groundwater. The results in Fig. 4(c) shows the lowest operating costs for Al1-Al2-Al3-Al4; Fe1-Fe2-Fe3-Fe4; Al1-Al2-Fe3-Al4 and Fe1-Fe2-Al3-Fe4as Rs. 3.44, 1.55, 2.07 and 2.90 Rs./m3for 45 min ET respectively. An electrode combination of Fe1-Fe2-Al3-Fe4(SA/V 40 m2/m3) for 16V and 0.67A for 45 min of ET was considered as the best electrode arrangement giving a small operating cost of 2.90 Rs./m3 for simultaneous removal of fluoride and arsenic.
Electrical energy consumption is calculated for the aforesaid different electrode arrangements for both arsenite and fluoride removal simultaneously using the equation(29) which was also used by24 for study carried out for treatment of paper mill effluent in batch stirred electrochemical tank reactor.
![]()
where, V is the applied cell voltage in volts, I is the current in ampere (A) and t is the treatment time in hours. As observed from Fig. 4(d) and Fig. 4(e), the energy consumption and % arsenite and fluoride removal for four different electrode combinations were 1.83, 1.98, 2.04 and 2.01 KWh/m3 for simultaneous removal of As(III) as 33%, 93%,92% and 96.5% and of 93%, 1.4%,65% and 100% respectively.
Therefore, it was concluded that with one single ECCtreatment for an electrode combinationFe1-Fe2-Al3-Fe4(SA/V: 40 m2/m3) in a bipolar arrangement providing simultaneous removal of both As(III) and F– at a meagre cost was achieved at 45 min ET and a good potable quality water. The water quality can further be improved by providing a small filtration unit down-line the ECC unit to remove small turbid particles if any.
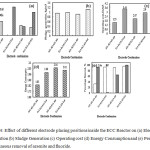 |
Figure 4: Effect of different electrode placing positions inside the ECC Reactor on (a) Electrode Dissolution (b) Sludge Generation (c) Operating cost (d) Energy Consumption and (e) Percentage simultaneous removal of arsenite and fluoride. |
Operating conditions: Bipolar arrangement; No. of plate electrodes: 4; As(III)o:1.6 mg/L; F–o: 12 mg/L; Al3+: 0 mg/L; Feo: 0.061 mg/L; Inter electrode spacing: 5 mm; Applied cell voltage: 16 V; SA/V ratio: 40 m2/m3 and Electrolysis time: 45 min.
Conclusion
ECC studies were carried out for simultaneous removal of both fluoride and arsenic from groundwater using Al and Fe plate electrodes for its placing position in the ECR. Preceded by a large set of experiments on electrode combinations using Fe and Al, 4 set of experiments with selected electrode combinations: (i) Al1-Al2-Al3-Al4 (ii) Fe1-Fe2-Fe3-Fe4 (iii) Al1-Al2-Fe3-Al4 and (iv) Fe1-Fe2-Al3-Fe4 were identified for detailed studies and carried out for an operating condition with bipolar arrangement, Electrodeno: 4,SA/V ratio: 40 m2/m3, pH: 7.99, As(III)o : 1.6 mg/L, F–o: 12 mg/L, Al3o+ 0 mg/L, Feo : 0.061 mg/L, Electrode spacing: 5 mm, cell voltage: 16 V, average current: 0.67 A, and 45 min ET. The following conclusions were drawn:
Fe1-Fe2-Al3-Fe4 electrode combination proved most effective and efficient for simultaneous removal of arsenite and fluoride from ground water. The concentration of arsenite and fluoride reduced to 0.04 mg/L and 0 mg/L at 30-45 min ET from its initial concentration of 1.6 mg/L and 12 mg/L.
Other drinking water quality parameters analyzed after ECC treatment were well within the drinking water standards prescribed by both BIS and WHO, with no addition of secondary pollutants into water. However, water quality after ECC for electrode combinations like Al1-Al2-Al3-Al4, Fe1-Fe2-Fe3-Fe4 and Al1-Al2-Fe3-Al4 were unsatisfactory because of inefficient removal of As(III) and F– and also residual iron and aluminum ions in water were noticed.
Cost wise economics for Fe1-Fe2-Al3-Fe4 showed a good combination with 0.667 kg/m3 of electrode dissolution, 1.115 kg/m3 of dry sludge generation, energy consumption of 2.01 KWh/m3; the overall operating cost being 2.90 Rs./m3 of water treated. For the operating conditions: bipolar Fe1-Fe2-Al3-Fe4; electrode spacing: 5 mm; pH0: 8.9; As(III)o: 1.6 mg/L; F–o: 12 mg/L; Al3+: 0 mg/L; Feo: 0.061 mg/L; inter-electrode spacing: 5 mm;E. no: 4; voltage: 16 Volts; average current: 0.66 A; SA/V ratio: 40 m2/m3 and an electrolysis time of 45 min.
These experimental results dictate the novelty of ECC removing both arsenite and fluoride simultaneously adsorbed to the co-precipitating with hydrous aluminum oxide and ferric oxide. The ECC process with Fe1-Fe2-Al3-Fe4 configuration signifies a note-worthy substitute/ a retrofit for treatment systems that operate separately for As(III) and F– removal, still retaining the beneficial water minerals in ground water as is.
The electrode placing position plays a very important role in the ECC treatment process for effective removal of both arsenite and fluoride from groundwater.This approach offsets the use of separate treatment technologies for removal of both arsenite and fluoride in one cost effective treatment with reduced capital cost, operation and maintenance costs and low energy footprints.
Acknowledgements
The authors are thankful to Sri Jayachamarajendra College of Engineering (JSS S&T University), My suru for giving the opportunity to carry out this research work.
References
- Nollet, L. M., Handbook of Water Analysis, 2nd edn. Ch.1. New York: CRC Press, 2007.
- Ehrenstein, O. S. V., Mazumder, D.N.G., Yuan, Y., Samanta, S., Balmes, J., Sil, A., Ghosh, N., Smith, M. H., Haque, R., Purushothaman, R., Lahiri, S., Das, S., and Smith, A. H., American Journal of Epidemiology, 2005, 162, 533-541.
- Grandjean, P., Olsen, J. H., Jensen, O.M., Juel, K. J., Cancer incidents and mortality in workers exposed to fluoride. National Cancer Institute (Bathesda), 1992, 84, 1903-1909.
- World Health Organization.,1993. Guidelines for drinking water quality: Remediations, Vol.1 (3rd edition). Geneva: WHO.
- IS: 10500, Bureau of Indian Standards, 1983.
- Hering, J. G., Chen, P. Y., Wilkie, J. A., Elimelech, M., Liang, S., J. Am. Water Works Assoc, 1996, 155-167.
- Li, Y., Zhang, F. S.,Xiu, F.R., Sci. Total Environ, 2009, 407, 5780-5786.
- Mohan, D and Pittman, C. U., J. Hazard. Mater, 2007, 142, 1-53.
- Chauhan,V. S.,Dwivedi, P. K., Iyengar, L, J. Hazard. Mater,2007, 139, 103-107.
- Mjengera, H and Mkongo, G., Phys. Chem. Earth, 2003, 28, 1097-1104.
- Kumar, S., Gupta, A., Yadav, J. P., Ind. J. Chem. Techn, 2007, 14, 355-361.
- Pinon-Miramontes, M., Bautista-Margulis, R. G., Perez-Hernandez, A., Fluoride,2003, 36, 122-128.
- Tahaikt, M., Achary, I., Menkouchi, S. M. A., Amor, Z., Taky, M., Alami, A., Boughriba, A., Hafsi, M., Elmidaoui, A., Desalination, 2006, 189, 215-220.
- Arora, M., Maheshwari, R. C., Jain, S. K., Gupta, A., Desalination, 2004, 170, 105-112.
- Hu, K and Dickson, J. M., J. Mem. Sci, 2006, 279, 529-538.
- Baird, R. B., Eaton, A. D., Rice, E. W., APHA, 2017
- Pletcher, D. and Walsh, F. C., Chapman and Hall, London, UK, 1990.
- Moudhen, G., Feki, M., Wery, M., Ayedi, H., J. Haz. Mat, 2008, 150, 124-135.
- Hung, H.C., Luke, C., Lu, Y.C., Sep. Purif. Technol., 2009, 65, 137-146.
- Bukhari, A. A., Abuzaid, N. S., Abdulappa, M. K., Essa, M. H.,Int. Fifth Saudi Engineering Conference, 1999, 3, 293-301.
- Ghosh, D., Solanki, H., Purkait, M. K., J. Haz. Mat., 2008, 155, 135-143.
- Hernandez, I. L., Diaz, C. B., Morales, G.R., Bilyeu, B., Nunez, F. U., Chem. Eng. J, 2009, 148, 97-105.
- Donini, J.C., Kan, J., Szynkarczuk, J., Hassan, T. A., Kar, K.L., Can., J. Chem. Eng., 1994, 72, 1007-1012.
- El-Ashtoukhy E-SZ., Amin, N.K., Abdelwahab, O, Chem. Eng. J, 2009, 146, 205-210.

This work is licensed under a Creative Commons Attribution 4.0 International License.









