Preparation of Cu in Se2 Thin Films by using Various Methods (A Short Review)
Ho Soonmin*1 , Sreekanth Mandati2, Ramkumar Chandran3, Archana Mallik4, Mohammad Arif Sobhan Bhuiyan5 and Deepa K. G6
, Sreekanth Mandati2, Ramkumar Chandran3, Archana Mallik4, Mohammad Arif Sobhan Bhuiyan5 and Deepa K. G6
1Centre for Green Chemistry and Applied Chemistry, INTI International University, Putra Nilai, 71800, Negeri Sembilan, Malaysia.
2Center for Solar Energy Materials, International Advanced Research Center for Powder Metallurgy and New Materials, Balapur PO, Hyderabad – 500005, Telangana, India.
3,4Electrometallurgy and Corrosion Laboratory, Department of Metallurgical and Materials Engineering, National Institute of Technology, Rourkela, Orissa, India.
5Electrical and Electronics Engineering, Xiamen University Malaysia, Jalan Sunsuria, Bandar Sunsuria, 43900 Sepang, Selangor, Malaysia.
6Interdisciplinary Centre for Energy Research, Indian Institute of Science, Bangalore 560012, India.
Corresponding Author E-mail: soonmin.ho@newinti.edu.my
DOI : http://dx.doi.org/10.13005/ojc/350101
Article Received on : 12-10-2018
Article Accepted on : 09-01-2019
Article Published : 14 Feb 2019
Cu In Se2 thin films are very important semiconductor material for solar cell applications because of chemical stability, direct band gap and high optical absorption coefficient. In this work, these films have been prepared by using different deposition techniques such as electrodeposition, solvothermal, vacuum evaporation, hydrothermal and pulsed electrode position technique. Cu In Se2 thin films were fully characterized by using field emission scanning electron microscopy, X-ray diffraction, Energy dispersive X-ray analysis, atomic force microscopy, UV-Visible spectrophotometer and Raman spectroscopy in order to study physical properties.
KEYWORDS:Copper; Cuinse2 Thin Films; Electro Deposition; Vacuum Deposition; Selanylideneindium; Selenium; Solar Cells
Download this article as:| Copy the following to cite this article: Soonmin H, Mandati S, Chandran R, Mallik A, Bhuiyan M. A. S. Deepa K. G. Preparation of Cu in Se2 Thin Films by using Various Methods (A Short Review). Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Soonmin H, Mandati S, Chandran R, Mallik A, Bhuiyan M. A. S. Deepa K. G. Preparation of Cu in Se2 Thin Films by using Various Methods (A Short Review). Orient J Chem 2019;35(1). Available from: https://bit.ly/2S15KZx |
Introduction
Thin films with thickness ranging from a few nanometres to several micrometres1-5 were synthesized by using different methods. Thin films have distinct advantages over bulk materials, such as they offer the potential for low cost processing with minimal material usage, and deposition could be carried out on various substrates.6-12 Different processes for depositing thin films are broadly categorized into physical and chemical methods. Chemical methods are comparatively easier and cheaper than physical methods. Among the chemical methods there are vapor based and wet chemical (solution based) methods.
Wet chemical method is again cheaper than chemical vapor based methods. In this, the material is synthesized (using precursor chemicals) by various methods such as spray pyrolysis, electrodeposition, spin coating, anodization, hydrothermal, solvothermal, sol-gel, co-precipitation and combustion. Usually these methods involve a reaction process with or without the assistance of thermal assistance. The reactions are supposed to occur mostly at atmospheric pressures or at a vacuum level less than 0.1 mbar. If necessary inert gas atmosphere will be created by evacuating the working chamber by a rotary pump.
Compared to physical methods, it is easy to control deposition parameters in chemical methods. The route could be chosen depending on the requirement and final application of the material. Methods such as successive ionic layer adsorption and reaction (SILAR),13-14 chemical bath deposition (CBD),15-20 spray pyrolysis,21-23 electrodeposition, spin coating, and anodization are used for direct growth of microcrystalline films onto the substrate. Meanwhile methods such as hydrothermal, sol-gel, co-precipitation, and combustion are used to prepare nanoparticles of the material which has to be made in the form of ink/paste by mixing suitable solvent followed by thermal treatment. In this work, deposition of CuInSe2 (CIS) films by using electrodeposition, solvothermal and hydrothermal methods was discussed. Characterization was performed by using various tools.
Literature Survey
Solvothermal Method
Solvothermal synthesis is a non-aqueous process in which the reaction between precursors occurs at higher pressure and at temperature [higher than the boiling of the solvent]. The reaction temperature is greater than 200°C in most of the cases. The phase and shape formation in CuInSe2 thin films (CIS) through this process depends on the parameters such as type of solvent, capping agent, reaction temperature and duration. The shape of the particle changes with change in the reaction temperature. Nanoparticles of chalcopyrite CIS were obtained from solvothermal reaction when ethylenediamine was used as solvent and capping agent. Usually, the reaction takes place at 180–220°C and the average reaction time was 16 h.24-28 In the meantime, nanorods were obtained with soft solvothermal reaction at 120°C with ethylenediamine as solvent.29 Microwave assisted solvothermal method with polyethylene glycol as solvent at above 200°C showed existence of multiple binary phases which requires high temperature annealing (~500°C).30 Table 1 shows the reaction temperature, time and the obtained shape of the nanoparticles. From the table 1, it is clear that, with the same solvent different morphology is obtained at different deposition durations and temperatures.
Table 1: Morphology of CuInSe2 with respect to different deposition conditions.
| Synthesis temperature (°C) | Solvent | Time (h) | Morphology | References |
| 180 | Ethylenediamine, ethanol | 2 | Dispersive plates | 31 |
| 180 | Ethylenediamine, ethanol | 12 | Microspheres | 31 |
| 180 | Ethylenediamine, ethanol | 48 | Interconnected sheets | 31 |
| 180 | Ehylenediamine | 18 | Sheets | 27 |
| 120 | Ethylenediamine | 18 | Nanorods | 28 |
| 200 | Ethylenediamine | 24 | Irregular | 26 |
| 200 | Ethylenediamine | 48 | Mixture of sphere, rod and belt-like structures | 26 |
| 180 | Diethylamine | 36 | Spheres | 24 |
| 180 | Ethylenediamine | 15 | Whiskers | 24 |
| 180 | Ethanol | 48 | Spheres | 32 |
Hydrothermal Method
In hydrothermal synthesis, as the name implies, the precursors are subjected to the reaction in water. In this method, an autoclave is often used to create an atmosphere of increased pressure which in turn reduces the activation energy of the formation of final product. Hydrothermal process is known to be eco-friendlier than the solvothermal method since it uses water as solvent. Water is cheaper than other solvents and it can act as a catalyst for the formation of desired materials by tuning the temperature and the pressure. Also the water could be removed very easily from the final product. Compared to solvothermal, hydrothermal reactions happen at low temperatures and low cost precursors are used.
In hydrothermal synthesis of CuInSe2 thin films (CIS) at 150°C for 2 h, when ethylenediamine was used as capping agent, wurtzite structured CIS was formed. Also different morphologies were obtained for different molarities of ethylenediamine. At 10 mol ratio of the capping agent leads to cube like morphology while 5 mol ratio leads to the formation of rod like morphology.33 Chalcopyrite nanocubes of CIS were obtained with hydrothermal synthesis at 180 °C for 20 h.34 Jang and co-workers synthesized chalcopyrite CIS nanoparticle from metal precursors which is dissolved in acetic acid.35 The reaction temperature of the autoclave was controlled from 180°C to 220°C for 8h to 16 h. At lower concentrations of acetic acid (< 5 M), CIS phase coexisted with CISe phase. For the hydrothermal method, triethanolamine (TEA) is used mainly as the chelating agent.36,37,38 Table 2 shows a review of deposition condition details of CIS using hydrothermal method and the resulting morphology. The morphology of sample strongly depended on the synthesis temperature, time, and chelating agent.
Table 2: Morphology of Cu In Se2 with respect to different hydrothermal deposition conditions.
| Synthesis temperature (°C) | Chelating agent | Time (h) | Morphology | References |
| 150 | Ethylenediamine | 2 | Spheres | 33 |
| 180 | — | 20 | Nanocubes | 34 |
| 180 | Acetic Acid | 12 | Spheres | 35 |
| 220 | Triethanolamine | 6 | Sheets | 36 |
| 180 | Triethanolamine | 3 | Sphere | 39 |
| 180 | Ethylenediamine | 0.30 | Rod | 40 |
Electrodeposition Technique
Electrodeposition is a method which also has been used widely in commercial sector. The elements to be deposited will be dissolved in a suitable electrolyte and the dissolved cations will be reduced with the help of applied bias so that they will form uniform coating on the working electrode (fig.1). In addition, the complexing agent may be included to bring the reduction potentials of the individual elements closer. This low-temperature process is reasonably fast which helps in the deposition of large number of samples in a short period. Aqueous or non-aqueous solvents could be used as electrolyte. Here the applied bias should be greater than the reduction potential of the material to be deposited.
CuInSe2 thin films (CIS) have been prepared extensively by electrodeposition. One-step and three-step electrodeposition are employed for the deposition of CIS. In one-step deposition the metal salts are dissolved in the electrolyte and compound will be deposited on the anode electrode in a single run. In three-step deposition, individual materials are deposited by one by one. Three-step process offers the feasibility to choose the applied bias, electrolyte and pH individually according to the reduction potential of the individual element. Meanwhile in one-step process, these parameters are selected which will be suitable for all the materials to be deposited.
As far as the film properties are concerned, as-deposited films in one-step process are found to be crystalline with CIS phase while three-step process requires further heat treatment to get CIS phase.41-45 The standard reduction potentials of Cu2+ and In3+ and Se4- are +0.1 V, -0.58 V and 0.5 V versus saturated calomel electrode (SCE), respectively.46 Hence, the deposition potential is found to vary between -0.6 and -0.9 V in these experiments so that all the elements will be reduced simultaneously. pH of the solution is maintained at ~ 2 in most of the electrodeposition process as mentioned in the table 1. The reduction potentials of individual elements are found to be different at different pH values thereby affecting the deposition potentials. Lower pH values cause the dissolution of deposited metals and slow down the formation and absorption of metal hydroxide. In the meantime, higher pH values slow down the dissolution of the deposited metals.47 Also In (indium) content in the film is low at higher pH because of the absorption of indium oxide in the electrolyte. Hence as a compromise between the reduction potentials a pH~ 2 is used in most of the experiments.
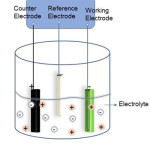 |
Figure 1: Electrodeposition set-up. |
Table 3: Various deposition conditions have been reported by different researchers.
|
pH |
Deposition potential (V) | Annealing temperature (°C) | References |
| 2 | -0.8 | 250 | 41 |
| 1.2 | -0.6 | 400 | 47 |
| 2.5 | -0.8 | 400 | 47 |
| 1.5 | -0.7 | 300 | 48 |
| 1.7 | -0.6 | 400 | 49 |
| 2.5 | -0.55 | 450 | 50 |
| 2 | -0.8 | 250 | 51 |
| 1.5 | -0.67 | 350 | 52 |
| 2 | -0.7 | 350 | 53 |
| 2 | -0.9 | 200 | 54 |
Secondary phases are quite normal in the as deposited films, which is removed by annealing. Annealing temperature varied from 250 to 450°C as indicated in Table 3. The shape of the grains in the CIS films formed by electrodeposition are found to have flower like structures.48-52
Electrodeposition of CISe in Aqueous and Non-Aqueous Electrolytes
The standard equilibrium reduction potential of Cu, In and Se ions in the electromotive force series (EMF) series is + 0.337/standard hydrogen electrode (SHE), − 0.342/SHE and + 0.741/SHE respectively. The challenge arises during electrodeposition of CISe is due to the active standard reduction potential of In (Indium) ions. Using complexing agents, the reduction potential of noble ions (Cu) can be shifted towards active ions (In), hence making the co-deposition of all the three ions easier. They also diminish hydrogen generation, avoid pinholes and improve the compactness of the as-deposited film. Other additives such as supporting electrolytes, surfactants, and brighteners are often used for improving the film quality.
The following section will briefly review the attempts made recently to co-deposit CISe films. Bhattacharya et al. were the first to report on the possibility to electrodeposit CISe thin films in a single step using triethanolamine (TEA) as complexing agent.55 Since then, several works on electrodeposition of CISe using TEA have been reported as it can form strong metal-complexes with Cu2+ and HSeO2- ions and weak complexing with the In 3+ ions. The most popularly used complexing agent in the early stages of CIS electrodeposition is thiocyanate ions (CNS–) due to its selectivity in the formation of ligands only with the Cu2+ ions.56,57,58 Other alternative complexing agents such as citric acid, trisodium citrate,59,60 tartrate ions,61 oxalic acid,62 glycine,63 potassium sodium tartrate64 and EDTA65 are also successfully utilized in the CISe electrodeposition. Apart from aqueous electrolytes, electrodeposition of CISe have been reported using non-aqueous electrolytes such as alcohol,66 ethylene glycol,67 DMF,68 DMSO69 and ionic liquids (reline).70 The availability of wide electrochemical deposition window in non-aqueous electrolytes allows electrodeposition of In (III) and Ga (III) ions conveniently without the interference of hydrogen evolution reactions. Unlike aqueous electrolytes (where the metal ions solubility is limited by oxides/hydroxides precipitation), high solubility, and ligand concentrations can be achieved in water-free ionic liquids. Moreover, the high ligand concentrations ensure greater control on the metal speciation in the electrolyte.71 Therefore, reline forms strong metal complexes with the ions, and hence, the necessity of using complexing agents is eliminated. Due to these reasons, non-aqueous electrolytes have gained significant attentions in the recent years.
Challenges In Obtaining Compositional CISe Thin Films with Pinhole Free and with Reduced Surface Non-Uniformities
The influential parameters such as deposition potential, pH and bath composition etc., which can directly affect the film quality and composition have been discussed in the several cited literatures.72-75 The stoichiometry and quality of the as-deposited CISe thin films mainly depends upon the deposition potential and [Se4+/Cu2+] ions flux ratio. An extensive study on the influence of ion flux ratio on the stoichiometry and phase formations for binary Cu-Se and ternary Cu-In-Se systems were reported by Thouin et al.76,77
However, for obtaining seamless CISe thin films by electrodeposition, critical issues such as surface non-uniformities, pinholes and erosion or dissolution of CISe film during electroplating in highly concentrated baths yet need further attention. Only few studies have been carried to avoid surface non-uniformities and pinholes in the as-deposited CISe thin films. Chandran et al,78 reported that severe surface non-uniformities along with severe pitting over the CISe thin films when higher In3+ ion concentrations were used. Fig.2 shows electrodeposited CISe using 10 mM of In3+ ions at a pH of 1.75 for 10 minutes. The as-deposited CISe film surface was highly In-rich and silvery by appearance. The presence of In- nano islands over the surface of the CISe films were confirmed by the image analysis as shown in fig 2a. After pre-treatment process, the FE-SEM of PT–CISe revealed the presence of uniform particle morphology with reduced In-nano-islands (Fig.2b).78
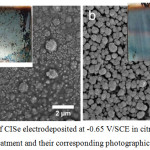 |
Figure 2: FE-SEM of CISe electrodeposited at -0.65 V/SCE in citrate bath with (a) and without (b) pre-treatment and their corresponding photographic images (inset).78 |
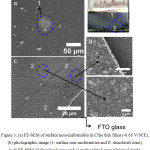 |
Figure 3: (a) FE-SEM of surface non-uniformities in CISe thin films (-0.65 V/SCE), (b) photographic image (1- surface non-uniformities and 2- dissoluted zone), (c-d) FE-SEM of dissoluted zone and e) undissoluted zone (Original work). |
Though employing optimal In3+ concentration and pre-treatment process can circumvent these issues, it was not possible to complete eliminate these surface non-uniformities completely. Fig. 3 shows the morphology of CISe deposited using less In3+ ion concentrations (5 mM). The compositional analysis revealed that the patches were majorly composed of Cu-poor CISe and rests of the other areas were Cu-rich. The plausible explanations for the appearance of such patches in CISe films can be due to the depletion or mass-controlled deposition of free Cu2+ ions along with fluctuating deposition of Cu-citrate complexes contributing to such Cu-poor patches in the CISe films. Such issues were minimized when high or optimal Cu2+ ion in the electrolyte & using fresh electrolytes for deposition. For prolonged deposition time (>15 min, pH=1.85), Chandran et al observed the dissolution of CISe thin films at the edges as shown in fig.3 (b,c). In fig.3(d), the microstructure of the dissoluted zone is provided and compared with undissoluted area (Fig.3e). The presence of platelet structures, a characteristic structure for Cux Se binary phase is evident for the dissoluting behavior of the CISe films, a similar mechanism taking place in binary Cu-Se system at higher cathodic potential.79,80 Tentatively, they speculate that this dissolution might be due to the depletion of Cu2+ ions and along with high diffusion current contributed from the In3+ ions. These effects were minimized when suitable surfactants were used in the deposition. For an optimized bath, they were able to obtain a Cu-poor near stoichiometric CISe with silver gray appearance with reduced surface non-uniformities. In fig.4, the possible strategies for obtaining quality CISe thin films are proposed. These preliminary studies can shed light in the development towards the surface non-uniformity free CISe thin films which can contribute near to the improvement in the final efficiency of the devices.
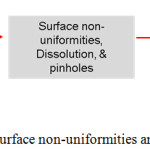 |
Figure 4: Strategies to avoid surface non-uniformities and pinholes in CISe thin films. |
Thermal Evaporation Method
Thermal evaporation is referred as one of the best processes after considering all aspects of fabrication and performance. It belongs to physical vapor deposition (PVD) category which uses vacuum technology for depositing a pure material to the desired surface. The materials to be deposited on substrate, (in this technique), can be atomic elements or molecules. The process encompasses warming the material to be deposited within a vacuum compartment to raise a vapor cloud inside the chamber. This vapor cloud navigates the compartment and sticks to substrate as a film.81 This heating can be either filament evaporation or E-Beam evaporation as illustrated in Figure 5.
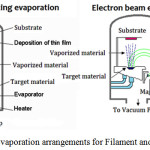 |
Figure 5: Evaporation arrangements for Filament and E-Beam. |
The prime advantage of the vacuum evaporation is to deposit high-purity films from high-purity source material. Moreover, a solid in any form and purity can be utilized as source material and allows the usages of masks to deposit on the desired portion of the substrate. Furthermore, monitoring and control of the deposition are also relatively easy. On the other hand, the main disadvantage of evaporation process is the deposition of compounds and alloy are difficult to control. Moreover, large vacuum chambers are generally required to keep the substrate safe.
Among numerous materials for thin film solar cells, copper indium diselenide films (CIS) have been identified as one of the most encouraging contestant as it has comparatively a better and stable conversion efficiency of 21.7 %.82 Due to its ideal adjustable band gap and high absorption, it has great electrical and optical characteristics. Furthermore, CIS adopts an easy and cost effective procedure to achieve large films at normal temperature.83,84,85 Thermal evaporation technique has been used to produce CuInSe2 films. Characterization has been performed (Table 4) by suing various tools such as X-ray diffraction (XRD), atomic force microscopy (AFM), Energy dispersive X-ray analysis (EDAX), scanning electron microscopy (SEM) and UV-Visible spectrophotometer.
Table 4: There are some highlighted results as reported by many researchers.
| References | Highlighted Results |
| Shah and co-workers, 200986 | XRD data confirmed single phase of CuInSe2 when the temperature was 423 K and above.Band gap values (1.01 to 1.06 eV), activation energy (82-42 meV), and electrical resistivity values (0.15-20 ohmcm), strongly depended on the deposition temperature. |
| Ray and co-workers, 200987 | EDAX spectra showed that near stoichiometric composition for all the samples deposited at various substrate temperatures (423-573 K). As the substrate temperature increases, the grain size was also increased according to atomic force microscopy images. |
| Prabahar and co-workers, 201088 | Thin films of various thicknesses (405, 565, 745 nm) have been prepared according to SEM analysis. The SEM images revealed that grain size is mainly depended on Cu:In ratio. |
| Hasan co-workers89 | The optical transmittance spectra confirmed that the films annealed at 350 °C for 15 minutes have been observed to have better qualities if compared to 200, 250 and 400 °C (do not show any well-defined absorption region). From the obtained band gap values (0.967, 0.975, 0.978 eV), we can observe that band gap is inversely proportional to the Cu/In ratios (0.958, 0.632, 0.579). |
| Mahesh co-workers90 | Copper indium diselendie films prepared on glass substrate (under vacuum 10-5 Torr) indicated a uniform and homogeneity distribution of crystallites. XRD data showed that annealed films are polycrystalline in nature. |
| Parihar and co-workers, 201191 | Current-voltage analysis showed better ideality factor values in annealed films (with increased film thickness). The Schottky diodes displayed the existence of barrier inhomogeneity at the M-S interface. |
| Senthil and co-workers, 199992 | Electronic hopping plays a great role as shown in conduction studies. The value of dielectric constant (10.8 at 5MHz and room temperature), ac (0.12 eV) and dc (0.27 eV) activation energies has been reported. |
Pulse electrodeposition
Pulse and pulse-reverse plating are the advanced features of electrodeposition possessing additional variables which can control aspects like composition and morphology of the electrodeposit. Pulse deposition involves parameters like cathodic potential/current, pulse on-time, pulse off-time and deposition time and pulse-reverse plating additionally has a reverse anodic potential/current with anodic pulse on-time and off-time. The parameters and features of pulse and pulse-reverse electrodeposition are schematically represented in Figure 6. Appropriate modulation of these parameters helps in improved control over composition of individual elements. In addition, diffusion of entrapped gases, rearrangement of ad-atoms, etc. take place during pulse off-time thereby leading to homogeneous compact deposition with reduced porosity.93
Pulse plating essentially plays a key role in deposition of ternary systems wherein achieving desired composition is crucial in final performance of the absorber layer. For instance, pulse off-time (relaxation time) during pulse electrodeposition can be suitably varied to obtain desirable composition of In in CuInSe2 (CIS) films.94 On the other hand, pulse-reverse electrodeposition, by virtue of possessing an additional anodic potential step helps in removal of impurities, surface dispersed undesired phases, etc. while also felicitating the advantages of pulse plating. Despite possessing additional process parameters and added advantages, pulse and pulse-reverse plating techniques are less explored for fabrication of CIS films.
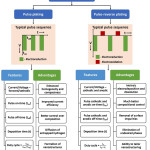 |
Figure 6: Schematic illustration of types, features and advantages of pulse electrodeposition. |
There are many research findings were reported by different scientists (Table 5). 1.42 % efficient CIS solar cells are prepared by Kang et al. wherein CIS absorber layers are fabricated using pulse-reverse electrodeposition technique with a subsequent selenization process.95 Caballero-Briones et al employed pulse electrodeposition for the preparation of CIS films and inferred that the films contain binary and ternary phases as observed from Raman spectroscopy analysis.96 Uniformly adhered CIS films with tetragonal chalcopyrite structure are fabricated using pulse plating technique wherein a bell-like modified square wave form was employed for deposition.97 Valdes et al reported the fabrication of chalcopyrite CIS films with different composition and morphologies by adopting a variable potential pulse sequence.98,99 Multi potential pulse electrodeposition was employed by Hu et al to fabricate single phase chalcopyrite CIS films and the different potentials in the study contributed to regulate the composition and homogeneity of deposition.100 The relative content of In was modulated appropriately by modifying the duty cycle during the pulse electrodeposition of CIS films and the study also unveiled a novel flake-like nanostructured morphology which aided in improved photoelectrochemical performance.101 Based on various studies, it is indeed clear that pulse electrodeposition adds the extra advantage in modulating the composition of individual elements and obtaining the stoichiometric CIS with chalcopyrite phase which is crucial in ternary compounds and eases the process of optimization for absorber layers.102-105
Table 5: Summary of reports on pulse electrodeposition of CuInSe2 thin films.
| Material | Method | Description | Performance | References |
| CuInSe2 | Pulse reverse electrodeposition and selenization | Stoichiometric copper poor CIS films by appropriate use of anodic potential | 1.42 % efficient CIS solar cells | Kang and co-workers, 200995 |
| CuInSe2 | Three step pulsed electrodeposition | Number of pulses, pulse duration and concentration are varied to obtain CIS films and as-deposited films are characterized | A photocurrent of 3.73 µA at -0.2 V | Caballero and co-workers, 201196 |
| CuInSe2 | Pulsed electrodeposition with multi potentials followed by annealing | Use of multi potential to obtain control over atomic ratios in CIS films | – | Hu and co-workers, 2011,100 |
| CuInSe2 | Pulsed electrodeposition and annealing | Optimization of In content by varying duty cycle. Chalcopyrite CIS films with nanostructured flake-like morphology | Photoactivity of CIS is confirmed from photoelectrochemical characterization | Mandati and co-workers, 2013101 |
| CuInSe2 | Pulse plating followed by annealing | Systematic study of CIS with varied duty cycle | Photoresponse from semiconductor-liquid junction | Mandati, 2015106 |
| CuInSe2 | Sequential potential pulses during electrodeposition | Improvement in microstructure and elimination of secondary phases with varied pulse duration and precursor concentration | Photoresponse and confirmation of p-type conductivity | Palacios-Padrós, and co-workers, 2010107 |
Conclusion
This short review has provided some of the progresses in the preparation of ternary CuInSe2 thin films by using solvothermal, hydrothermal, electrodeposition, vacuum evaporation and pulsed electrodeposition method. The advantage of disadvantage of each technique has been briefly described. Critical issues pertaining to the surface non-uniformities and strategies to avoid the issues have been proposed. Currently, power conversion efficiency more than 10 % could be observed for the CuInSe2 films.
Acknowledgements
This work is supported by Inti International University (HO SM).
References
- Al-Rashedi, K., Farooqui, M., Rabbani, G. (2018). Charactering crystalline chromium oxide thin film growth by sol-gel method on glass substrates. Oriental Journal of Chemistry, 34, DOI : http://dx.doi.org/10.13005/ojc/3404064.
- Meena, S.P., Ashokkumar, R. (2018). Effect of different current density on properties of electrode posited NiCoCr thin films. Oriental Journal if Chemistry, DOI : http://dx.doi.org/10.13005/ojc/3404023.
- Anandh, B.A., Ganesh, A.S., Thangarasu, R., Sakthivel, R., Kannusamy, R., Tamilselvan, K., (2018) Structural, morphological and optical properties of aluminium doped ZnO thin film by dip coating method. Oriental Journal of Chemistry, 34, DOI : http://dx.doi.org/10.13005/ojc/340356.
- Ho, S.M. (2017). Study of structural properties of Ni3Pb2S2 films. Oriental Journal of Chemistry, 33, DOI : http://dx.doi.org/10.13005/ojc/330466.
- Sabaghi, M., Majdabadi, A., Marjani, S., Khosroabadi, S., (2015) Optimization of high efficiency CdS/CdTe thin film solar cell using step doping grading and thickness of the absorption layer. Oriental Journal of Chemistry, 31, DOI : http://dx.doi.org/10.13005/ojc/310232.
- Ahmad HJ, Anuar K, Ho SM, Tan WT, Abdul HA (2010) Effect of solution concentration on MnS2 thin films deposited in a chemical bath. Kasetsart Journal: natural Science, 44, 446-453.
- Ho SM (2014) Chemical bath deposited lead sulphide thin films: preparation and characterization. World of Mechanics, 1,1-6.
- Atan S, Anuar K, Ho SM, Jelas H (2011) The effect of the pH value on the growth and properties of chemical bath deposited SnS thin films. Research Journal of Chemistry and Environment, 15, 45-48.
- Ho SM (2015) Scanning electron microscopy study of surface morphology of Ni3Pb2S2 thin films. Asian Journal of Chemistry, 27, 3851-3853.
- Kassim A, Ho SM, Tan WT, Nagalingam S, Monohorn S (2010) Effect of bath temperature on the chemical bath deposition of PbSe thin films. Kathmandu University Journal of Science, Engineering and Technology, 6, 126-132.
- Lim KS, Ho SM, Anuar K, Saravanan N (2013) Investigation of morphological properties of the copper sulphide films in acidic media based on atomic force microscopy. International Research Journal of Chemistry, 3, 62-68.
- Noraini K, Kassim A, Ho SM, Abdul HA (2010) Influence of the deposition time on the structure and morphology of the ZnS thin films electrodeposited on indium tin oxide substrates. Digest Journal of Nanomaterials and Biostructures, 5, 975-980.
- Aykut A (2016) Structural and optical characterization of Cu2SnSe3 thin films prepared by SILAR method. Thin Solid Films, 615, 324-328.
- Mukherjee, A., Satpati, B., Bhayyacharyya, S.R., Ghosh, R., Mitra, P. (2015). Synthesis of nanocrystalline CdS thin film by SILAR and their characterization. Physica E: Low dimensional Systems and Nanostructures, 65, 51-55.
- Loh YY, Ho SM, Anuar K, Saravanan N (2010) Structural and morphological characterization of chemical bath deposition of FeS thin films in the presence of sodium tartrate as a complexing agent. Silpakorn University Science and Technology Journal. 4, 36-42.
- Mohd YR, Mohd JH, Anuar K, H SM, Tan WT, Abdul HA, Saravanan N (2010) Chemical bath deposition of NiSe thin films from aqueous solution. Kuwait Journal of Science and Engineering, 37, 63-73.
- Sharif AM, Anuar K, Ho SM, Tan WT, Abdullah DK, Gwee SY (2010) Preparation and characterization of iron sulphide thin films by chemical bath deposition method. Indonesian Journal of Chemistry, 10, 8-11.
- Saravanan N, Anuar K, Ho SM, Tan WT (2011) Influence of pH on the properties of chemical bath deposited Ni4S3 thin films. Bangladesh Journal of Scientific and Industrial Research, 46, 243-246.
- Tan WT, Ho SM, Anuar K, Kelvin (2011) Composition, morphology and optical characterization of chemical bath deposited ZnSe thin films. European Journal of Aplied Sciences, 3, 75-80.
- Gopinath, G.R., R.W. Miles and K.T. R. Reddy, 2013. Influence of bath temperature on the properties of In2S3 films grown by chemical bath deposition. Energy Procedia, 34: 399-406.
- Kamoun N, Bouzouita, H., Rezig, B. (2007). Fabrication and characterization of Cu2ZnSnS4 thin films deposited by spray pyrolysis technique. Thin Solid Films, 515, 5949-5952.
- Teny, T.J., Meril, M., Kartha, C.S., Abe, T., Vijayakumar, K.P., Kashiwaba, Y. (2005). CuInS2/In2S3 thin film solar cell using spray pyrolysis technique having 9.5 % efficiency. Solar Energy Materials and Solar Cells, 89, 27-36.
- Elidrissi B, Addou M, Reggragui M, Bougrine A, Kachouane A, Bernede JC (2001) Structure, composition and optical properties of ZnS thin films prepared by spray pyrolysis. Materials Chemistry and physics, 68, 175-179.
- Li, Bin, Xie, Yi, Huang, Jiaxing., & Qian, Yitai., (1999). Synthesis by a solvothermal route and characterization of CuInSe2 nanowhiskers and nanoparticles. Adv. Mater. 11, 1456–1459.
- Kim, Ki-Hyun, Chun, Young-Gab, Park, Byung-Ok, Yoon, & Kyung- Hoon, (2004). Synthesis of CuInSe2 and CuInGaSe2 nanoparticles by solvothermal route, Mater. Sci. Forum, 449–452, 273–276.
- Chen, Huiyu, Yu, Seong-Man, Shin, Dong-Wook, Yoo, Ji-Beom, (2010). Solvothermal synthesis and characterization of chalcopyrite CuInSe2 nanoparticles, Nanoscale Res. Lett. 5, 217–223.
- Luo, P., Yu, P., Zuo, R., Jin, J., Ding, Y., Song, J., & Chen, Y., (2010). The preparation of CuInSe2 films by solvothermal route and non-vacuum spin-coating process, Physica B, 405, 3294–3298.
- Yi-Han, Y., & Yit-Tsong, C., (2006). Solvothermal preparation and spectroscopic characterization of copper indium diselenide nanorods, J. Phys. Chem. B, 110, 17370–17374.
- Jeong, S., Lee, B-S., Ahn, S., Yoon, K-H., Seo, Y-H., Choia, Y., & Ryu, B-H., (2012). An 8.2% efficient solution-processed CuInSe2 solar cell based on multiphase CuInSe2 nanoparticles, Energy Environ. Sci., 5, 7539–7542.
- Pulgarin-Agudelo, FA., Placidi, M., Fairbrother, A., Fontané X., Roca, VO., Sebastian, PJ., Ramos, F., Pina, B., Pérez-Rodríguez, A., & Saucedo, E., (2013). Synthesis of CuInSe2 nanopowders by microwave assisted solvothermal method, International Journal of Nanotechnology, 10(12), 1029–1044.
- Zhang L, Liang J, Peng SS, Chen J (2007) Solvothermal synthesis and optical characterization of chalcopyrite CuInSe2 microspheres, Materials Chemistry and physics, 106, 296-300.
- Liu K, Liu H, Xu Y, Zhang L (2016) The effects of different morphologies on synthesis of CuInSe2 prepared by co-reduction. J Appl Biomater Funct Mater, 14, S77-S79.
- Ramkumar, J., Ananthakumar, S., & Babu,SM., (2014). Hydrothermal synthesis and characterization of CuInSe2 nanoparticles using ethylenediamine as capping agent, Solar Energy, 106, 177–183.
- Sugan, S., Baskar, K., & Dhanasekaran, R., (2014). Hydrothermal synthesis of chalcopyrite CuInS2, CuInSe2 and CuInTe2 nanocubes and their characterization, Current Applied Physics, 14, 1416-1420.
- Shim, JB., Kim, CG., Jeon,DJ., Chung, T-M., An, KS., Lee, SS., Lim, JS., Jeong, SJ., Park, BK.,& Lee, YK.,(2013). Hydrothermal synthesis of CuInSe2 Nanoparticles in acetic acid, Journal of Physics and Chemistry of Solids, 74, 867–871.
- Jeng-Shin, M., Das, S., Yang, C-Y., Chen,F-S., & Lu, CH., (2014). Hydrothermally-assisted selenization of CuInSe2 thin films on copper foils, Ceramics International, 40, 7555–7560.
- Wu, CH., Chen, F-S., Lin, SH., Lu, & CH., (2011). Preparation and characterization of CuInSe2 particles via the hydrothermal route for thin-film solar cells, Journal of Alloys and Compounds, 509(19), 5783-5788.
- Patil, SV., Desai, ND., Suvarta, KD., Rahul, MM., & Poatrao, BN. (2017). Room Temperature Synthesis of Nanocubic CuInSe2 Thin Films, J Adv Chem Eng 7(1), 171.
- Chung W, Jeng M, Shin L, Chung L (2013) Synthesis of CuInSe2 thin films on flexible Ti foils via the hydrothermally assisted chemical bath deposition process at low temperatures. Solar Energy Materials and Solar Cells, 112, 47-51.
- Wu CC, Yeh S, Delele WA, Wei S, Ming C, Chiu C, Bing H (2010) Rapid microwave enhanced solvothermal process for synthesis of CuInSe2 particles and its morphologic manipulation. Chem Mater, 22, 4185-4190.
- Deepa, KG., Shruthi, NL., Sunil, MA., & Nagaraju, J. (2014). Electrodeposited Cu(In,Al)Se2 thin films for device applications, Thin solid films, 551, 1-7.
- Calixto, ME., Dobson,KD., McCandless, BE., & Birkmire, RW. (2006). Controlling growth chemistry and morphology of single-bath electrode posited Cu In GaSe2 thin films for photovoltaic application, Journal of The Electrochemical Society, 153 (6), G521-G528.
- Lee, B-S., Park, S-YL., Lee, JM., Jeong, JH., Kim,JY., Chung, C-H., & Lee, D-K., (2016). Suppressed Formation of Conductive Phases in One-Pot Electrodeposited CuInSe2 by Tuning Se Concentration in Aqueous Electrolyte. ACS Appl. Mater. Interfaces 8, 24585−24593.
- Saïdi,H., Boujmil, MF., Durand, B., Lazzari, J-L., & Bouaïcha, M. (2018). Elaboration and characterization of CuInSe2 thinfilms using one-step electrodeposition method on silicon substrate for photovoltaic application. Mater. Res. Express,5, 016414.
- Ramdani, O., Guillemoles, JF., Lincot, D., Grand, PP., Chassaing, E., Kerrec, O., & Rzepka,E. (2007). One-step electrodeposited Cu In Se2 thin films studied by Raman spectroscopy, Thin Solid Films, 515, 5909–5912.
- Kois, J., Bereznev, S., Mellikov, E., & Opik, A., (2006). Electrodeposition of CuInSe2 thin films onto Mo-glass substrates, Thin Solid Films, 511 – 512, 420 – 424.
- Ashwini, B. R., Priyanka, UL., & Chaure, NB., (2015). The effect of pH and selenization on the properties of CuInSe2 thin films prepared by electrodeposition technique for device applications, J Solid State Electrochem, 19, 201–210.
- Yadolah, G, Touradj, E, Mahmood, K, Amir, M., & Mansoor, K-R, (2015). Effect of pH on the electrodeposition of Cu(In, Al)Se2 from aqueous solution in presence of citric acid as complexing agent, Surface Review and Letters, 22, 1550057.
- Guillen, C., Galiano, E., & Herrero, J., (1991). Cathodic electrodeposition of CuInSe2 Thin Films, Thin Solid Films, 195, 137-146.
- Frontini, MA., & Vázquez, M., (2010). Electrodeposition of CuInSe2 in citrate-containing electrolytes, J Mater Sci, 45, 2995–3000.
- Chraibi, F., Fahoume, M., Ennaoui, A., &. Delplancke, JL., one step electrodeposition of CuInSe2 thin films, J. Condensed matter, 5, 88-96.
- B. Ndiaye, C. Mbow, M.S. Mane and C. Sène, One-step electrodeposited CuInSe2 absorber layers for efficient PV cells, Revue des Energies Renouvelables Vol. 15 N°4 (2012),609–620.
- Thou-Jen, W., Mu-Tao, H., Ya-Chun, K., & Shyan-Jer, L., (2009). A study of electrodeposition of CuInSe2 thin films with triethanolamine as the complexing agent, Applied Surface Science, 255, 4600–4605.
- Soon, HK., Yu-Kyung, K., Don-Soo, C., & Yung-Eun, S., (2006). Characterization of electrodeposited CuInSe2 (CIS) film, Electrochimica Acta, 51, 4433-4438.
- Bhattacharya, R. N., & Rajeshwar, K. (1986). Electrodeposition of CuInX (X= Se, Te) thin films. Solar Cells, 16, 237-243.
- Ganchev, M. G., & Kochev, K. D. (1993). Investigation of the electrodeposition process in the Cu-In-Se system. Solar energy materials and solar cells, 31(2), 163-170.
- Kemell, M., Ritala, M., & Leskelä, M. (2001). Effects of post-deposition treatments on the photoactivity of CuInSe2 thin films deposited by the induced co-deposition mechanism. Journal of Materials Chemistry, 11(2), 668-672.
- Tzvetkova, E., Stratieva, N., Ganchev, M., Tomov, I., Ivanova, K., & Kochev, K. (1997). Preparation and structure of annealed CuInSe2 electrodeposited films. Thin Solid Films, 311(1-2), 101-106.
- Thouin, L., Massaccesi, S., Sanchez, S., & Vedel, J. (1994). Formation of copper indium diselenide by electrodeposition. Journal of Electroanalytical Chemistry, 374(1-2), 81-88.
- Yang, J., Jin, Z., Li, C., Wang, W., & Chai, Y. (2009). Electrodeposition of CuInSe2 films by an alternating double-potentiostatic method using nearly neutral electrolytes. Electrochemistry Communications, 11(3), 711-714.
- Yang, M. H., Lee, M. L., Lin, Y. M., & Hwang, H. L. (1987). Determination of CuInSe2 thin film compositions by controlled-potential coulometry. Thin solid films, 155(2), 317-324 .
- Sun, J., Batabyal, S. K., Tran, P. D., & Wong, L. H. (2014). Electrodeposition of single phase CuInSe2 for solar energy harvesting: Role of different acidic additives. Journal of Alloys and Compounds, 591, 127-131.
- Ugarte, R., Schrebler, R., Cordova, R., Dalchiele, E. A., & Gomez, H. (1999). Electrodeposition of CuInSe2 thin films in a glycine acid medium. Thin Solid Films, 340(1-2), 117-124.
- Aksu, S., Wang, J., & Basol, B. M. (2009). Electrodeposition of In–Se and Ga–Se thin films for preparation of CIGS solar cells. Electrochemical and Solid-State Letters, 12(5), D33-D35.
- Aksu, S., & Pinarbasi, M. (2010, June). Electrodeposition of Cu-In-Ga films for the preparation of CIGS solar cells. In Photovoltaic Specialists Conference (PVSC), 2010 35th IEEE (pp. 000794-000798). IEEE.
- Long, F., Wang, W., Du, J., & Zou, Z. (2009). CIS (CIGS) thin films prepared for solar cells by one-step electrodeposition in alcohol solution. In Journal of Physics: Conference Series (Vol. 152, No. 1, p. 012074). IOP Publishing.
- Wellings, J. S., Samantilleke, A. P., Heavens, S. N., Warren, P., & Dharmadasa, I. M. (2009). Electrodeposition of CuInSe2 from ethylene glycol at 150 C. Solar Energy Materials and Solar Cells, 93(9), 1518-1523.
- Lai, Y., Liu, F., Zhang, Z., Liu, J., Li, Y., Kuang, S., Li, J. & Liu, Y. (2009). Cyclic voltammetry study of electrodeposition of Cu (In, Ga) Se2 thin films. Electrochimica Acta, 54(11), 3004-3010.
- Hung, P. K., Lin, T. H., & Houng, M. P. (2014). Characteristics of CuInSe2 Nanowire Arrays Electrodeposited into Anodic Alumina Templates with Dimethyl Sulfoxide (DMSO) Additive. Journal of The Electrochemical Society, 161(3), D79-D86.
- Harati, M., Jia, J., Giffard, K., Pellarin, K., Hewson, C., Love, D. A., Lau, W.M. , & Ding, Z. (2010). One-pot electrodeposition, characterization and photoactivity of stoichiometric copper indium gallium diselenide (CIGS) thin films for solar cells. Physical Chemistry Chemical Physics, 12(46), 15282-15290.
- Simka, W., Puszczyk, D., & Nawrat, G. (2009). Electrodeposition of metals from non-aqueous solutions. Electrochimica Acta, 54(23), 5307-5319.
- Chaure, N. B., Samantilleke, A. P., Burton, R. P., Young, J., & Dharmadasa, I. M. (2005). Electrodeposition of p+, p, i, n and n+-type copper indium gallium diselenide for development of multilayer thin film solar cells. Thin Solid Films, 472(1-2), 212-216.
- Chaure, N. B., Young, J., Samantilleke, A. P., & Dharmadasa, I. M. (2004). Electrodeposition of p–i–n type CuInSe2 multilayers for photovoltaic applications. Solar energy materials and solar cells, 81(1), 125-133.
- Saji, V. S., Choi, I. H., & Lee, C. W. (2011). Progress in electrodeposited absorber layer for CuIn (1− x) GaxSe2 (CIGS) solar cells. Solar Energy, 85(11), 2666-2678.
- Saji, V. S., Jung, C. Y., & Lee, C. W. (2015). Electrodeposition of copper, selenium, indium, and gallium on molybdenum/surface oxides: unary, binary, ternary and quaternary compositions. Journal of The Electrochemical Society, 162(9), D465-D479.
- Thouin, L., & Vedel, J. (1995). Electrodeposition and characterization of CulnSe2 thin films. Journal of The Electrochemical Society, 142(9), 2996-3001.
- Thouin, L., Rouquette-Sanchez, S., & Vedel, J. (1993). Electrodeposition of copper-selenium binaries in a citric acid medium. Electrochimica acta, 38(16), 2387-2394.
- Chandran, R., Panda, S. K., & Mallik, A. (2018). A short review on the advancements in electroplating of CuInGaSe2 thin films. Materials for Renewable and Sustainable Energy, 7(2), 6. http://doi.org/10.1007/s40243-018-0112-1.
- Chassaing, E., Ramdani, O., Grand, P. P., Guillemoles, J. F., & Lincot, D. (2008). New insights in the electrodeposition mechanism of CuInSe2 thin films for solar cell applications. physica status solidi c, 5(11), 3445-3448.
- Chandran, R., Pandey, R., & Mallik, A. (2015). One step electrodeposition of CuInSe2 from an acidic bath: A reduction co-deposition study. Materials Letters, 160, 275-277.
- Bhuiyan, M.A.S. & Mahmood, Z.H. (2008) Study of optical properties of CuInSe2 thin film. 2nd National Workshop on Advanced Optoelectronic Materials and Devices AOMD 2008, 129-133.
- Jackson, P., Hariskos, D., Wuerz, R., Kiowski, O., Bauer, A., Friedlmeier, T. M. & Powalla, M. (2014) Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Physics Status Solidi RRL., 1–4.
- Alageli, A.S. & Elsamahi, M.I. (2013) Effect of Film Thickness on the Structure and Electrical properties of CuInSe2 Thin Films. Applied Mechanics and Materials. 372, 579-585.
- Yakushev, M.V., Mudryi, A.V., Borodavchenko, O.M., Volkov, V.A. & Martin, R.W. (2015) A photoluminescence study of excitonic grade CuInSe2 single crystals irradiated with 6 MeV electrons. Journal of Applied Physics, 118, 155703–1-155703-7.
- Islam, M.A., Karim, A. M. M. T., Julkarnain, M., Badrul, A.K.M., Khan, M.K.R. & Khan, K.A. (2017) Opto-transport properties of e-beam evaporated annealed CuInSe2 thin films. Surfaces and Interfaces. 8, 170-175.
- Shah NM, Panchal CJ, Kheraj VA, Ray JR, Desai MS (2009) Growth, structural and optical properties of copper indium diselenide thin films deposited by thermal evaporation method. Solar Energy, 83, 753-760.
- Ray JR, Shah NM, Patel KJ, Kheraj VA, Desai MS, Panchal CJ, Rehani B (2009) Structural, electrical and optical properties of copper indium diselenide thin film prepared by thermal evaporation method. Thin Solid Films, 517, 3639-3644.
- Prabahar S, Balasubramanian V, Suryanarayanan N, Muthukumarasamy N (2010) Structural, characterization of vacuum evaporation copper indium diselenide thin films. Journal of Ovonic Research, 6, 45-50.
- Hasan SMF, Khaliquzzaman M, Biswas SK, Quadir L, Shariff MA, Rahman MM () Study of the composition of CuInSe2 and CuIn1-xGaxSe2 semiconductor thin films prepared by evaporation followed by annealing using RBS and PIXE and their properties. 117-126, https://inis.iaea.org/collection/NCLCollectionStore/_Public/33/034/33034264.pdf.
- Mahesh CS, Balram T, Vijay YK (2011) Optical characterization of CuInSe2 thin films prepared by vacuum thermal evaporation method. AIP Conference Proceedings, 1391, 737, https://doi.org/10.1063/1.3643664.
- Parihar U, Ray JR, Kumar N, Sachdeva R, Padha N, Panchal CJ (2011). Impact of annealing on CuInSe2 thin films and its Schottky interface. Journal of Nano and Electronic Physics, 3, http://essuir.sumdu.edu.ua/handle/123456789/27928.
- Senthil K, Nataraj D, Prabakar K, Mangalaraj D, Narayandass K, Udhayakumar N, Krishnakumar N (1999) Conduction studies on copper indium diselenide thin films. Materials Chemistry and Physics, 58, 221-226.
- Mandati, S., Sarada, B. V., Dey, S. R., & Joshi, S. V. (2013). Improved photoelectrochemical performance of Cu(In,Ga)Se2 thin films prepared by pulsed electrodeposition. Journal of Renewable and Sustainable Energy, 5, 031602.
- Mandati, S., Sarada, B. V., Dey, S. R., & Joshi, S. V. (2013). Pulsed Electrodeposition of CuInSe2 Thin Films with Morphology for Solar Cell Applications. Journal of the Electrochemical Society, 160, D173-D177.
- Kang, F., Ao, J., Sun, G., He, Q., & Sun, Y. (2009). Structure and photovoltaic characteristics of CuInSe2 thin films prepared by pulse-reverse electrodeposition and selenization process. Journal of Alloys and Compounds, 478(1–2), L25-L27. doi: http://dx.doi.org/10.1016/j.jallcom.2008.12.020.
- Caballero-Briones, F., Palacios-Padrós, A., & Sanz, F. (2011). CuInSe2 films prepared by three step pulsed electrodeposition. Deposition mechanisms, optical and photoelectrochemical studies. Electrochimica Acta, 56(26), 9556-9567. doi: http://dx.doi.org/10.1016/j.electacta.2011.06.024.
- Wang, X., Wang, G., Tian, B., Wan, S., & Du, Z. (2010). CuInSe2 thin films obtained by pulse-plating electrodeposition technique with novel pulse wave. Chinese Science Bulletin, 55(18), 1854-1858. doi: 10.1007/s11434-010-3185-5.
- Valdés, M., & Vázquez, M. (2012). Composition, morphology, and optical properties of CuInSe2 thin films electrodeposited using constant and pulsed potentials. Journal of Solid State Electrochemistry, 16(12), 3825-3835. doi: 10.1007/s10008-012-1821-5.
- Valdés, M. H., & Vázquez, M. (2011). Pulsed electrodeposition of p-type CuInSe2 thin films. Electrochimica Acta, 56(19), 6866-6873. doi: http://dx.doi.org/10.1016/j.electacta.2011.05.108.
- Hu, S.-Y., Lee, W.-H., Chang, S.-C., Cheng, Y.-L., & Wang, Y.-L. (2011). Pulsed Electrodeposition of CuInSe2 Thin Films onto Mo-Glass Substrates. Journal of the Electrochemical Society, 158(5), B557-B561. doi: 10.1149/1.3567762.
- Mandati, S., Sarada, B. V., Dey, S. R., & Joshi, S. V. (2013). Pulse Electrodeposition and Characterization of CIGS Thin-films for Solar Cell Applications. Paper presented at the Fifth ISEAC Triennial International Conference on Advances and Recent Trends in Electrochemistry, Hyderabad, India.
- Prasher, D., & Rajaram, P. (2012). Growth and characterization of pulse electrodeposited CuInSe2 thin films. Electronic Materials Letters, 8(5), 515-518. doi: 10.1007/s13391-012-2061-7.
- Shanmugavel, A., Srinivasan, K., & Murali, K. R. (2013). Pulse electrodeposited copper indium sulpho selenide films and their properties. Materials Science in Semiconductor Processing, 16(6), 1665-1671. doi: http://dx.doi.org/10.1016/j.mssp.2013.05.002.
- Fu, Y.-P., You, R.-W., & Lew, K. K. (2009). CuIn1−xGaxSe2 Absorber Layer Fabricated by Pulse-Reverse Electrodeposition Technique for Thin Films Solar Cell. Journal of the Electrochemical Society, 156, D553-D557.
- Liu, F., Huang, C., Lai, Y., Zhang, Z., Li, J., & Liu, Y. (2011). Preparation of Cu(In,Ga)Se2 thin films by pulse electrodeposition. Journal of Alloys and Compounds, 509, L129-L133.
- Mandati, S. (2015). Fabrication of CuInSe2 and Cu(In, Ga)Se2 Absorber Layers by Pulse-and Pulse-reverse Electrochemical Techniques for Solar Photovoltaic Applications, Physical Review D, 82(3), 1-49, doi: http://raiith.iith.ac.in/1413/.
- Palacios-Padrós, A., Caballero-Briones, F., & Sanz, F. (2010). Enhancement in as-grown CuInSe2 film microstructure by a three potential pulsed electrodeposition method. Electrochemistry Communications, 12(8), 1025-1029. doi: https://doi.org/10.1016/j.elecom.2010.05.015.

This work is licensed under a Creative Commons Attribution 4.0 International License.









