Low-Temperature Synthesis of α-Fe2O3/MWCNTS as Photo-Catalyst for Degradation of Organic Pollutants
Nuha Abdul Jaleel Omran, Zaid Hamid Mahmoud* , Noor Kadhum Ahmed and Farah Kefah Ali3
, Noor Kadhum Ahmed and Farah Kefah Ali3
Department of Chemistry, College of Science, Diyala University, Iraq.
Corresponding Author E-mail: zaidhameed_91@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/350140
Article Received on : 19-11-2018
Article Accepted on : 03-01-2019
Article Published : 01 Feb 2019
The nanoparticles of α-Fe2O3/ multi wall carbon nanotubes (MWCNTS) were successfully fabricated using photolysis and sonication method as a heterogeneous catalyst for photodegradation of 4-chlorophenol. The XRD and SEM were confirmed the supporting and distribution of the MWCNTS between the surface of α-Fe2O3 while, preparing nanoparticles proved also using XRD and SEM. The photocatalyic activity of prepared nanoparticles was investigated using 4-CP. The results appeared converting nearly 98% from 4-chlorophenol to 1,4- benzoquinone with optimum conditions of pH 3, 20ppm of pollutant and 0.3g of heterogeneous catalyst.
KEYWORDS:α-Fe2O3; Catalyst; Heterogeneous; Nanoparticles; Photolysis
Download this article as:| Copy the following to cite this article: Omran N. A. J, Mahmoud Z. H, Ahmed N. K, Ali F. K. Low-Temperature Synthesis of α-Fe2O3/MWCNTS As Photo-Catalyst for Degradation of Organic Pollutants. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Omran N. A. J, Mahmoud Z. H, Ahmed N. K, Ali F. K. Low-Temperature Synthesis of α-Fe2O3/MWCNTS As Photo-Catalyst for Degradation of Organic Pollutants. Orient J Chem 2019;35(1). Available from: https://bit.ly/2UydQu9 |
Introduction
4-chlorophenol is a hazardous and toxic organic pollutants so that the maximum concentration allowed in the water is in the range 1-20 ppb.1-4 To eliminate the negative impact, the advanced oxidation processes (AOPs) were suggested rather than a traditional biological treatment process because of high efficiency for degradation the dyes and organic pollutants by forming free radicals in the solution under effect UV irradiation.5,6 Under UV irradiation, heterogeneous catalysts work on excitation the electrons from the valence band (VB) to the conduction band (CB) leaving positive holes (h+) then, it initiates a series of oxidation-reduction reaction (redox) to product hydroxide and peroxyl free radicals as a strong oxidants to degrade the organic pollutants.7-12 However, use one catalyst in the process cause incomplete mineralization so it was modified by another material to increase the efficiency. This paper reported the new method13-16 to prepare heterogeneous catalysts. In this method, building of the nanoparticles takes bottom to top type. This means the UV radiation main role to build the particles from the solution by forming very small particles called seed then, the seed grows to form nanoparticles.
Materials and Method
Materials
4-chlorophenol, hydrochloric acid, sodium hydroxide, ferric chloride hexahydrate and Multiwall carbon nanotubes were supplied from Fluka company and used without purification.
Synthesis of α-Fe2O3 NPs
Firstly, in 250ml beaker, 1g (0.003mol) of FeCl3.6H2O dissolved in 100ml distilled water. Then, it transferred to the reactor of irradiation system as shown in fig 1. The solution of salt was irradiate until brown precipitate appears. After that, the precipitate separated, collected and washed using acetone and distilled water for 3 times. Finally, the precipitate dried and burned for 2hr.
 |
Figure 1: Irradiation system. |
Synthesis of α-Fe2O3/MWCNT Heterogeneous Catalyst
In a typical procedure, 2g (0.012mol) of nanorods were dispersed in 250ml of H2O2 containing 0.6g (0.05mol) of multi wall carbon nanotube (MWCNTS). Subsequently, the mixture was dispersed and ultrasonically for 30min until black-brown precipitate appears.
Degradation of 4-Chlorophenol Experiments
The photoactivity of heterogeneous catalyst prepared was investigated by degradation of 4-chlorophenol under ultraviolet irradiation (UV) with power 125W. All experiments were carried out using beaker with volume 250ml as a reactor at room temperature (2°C). The catalyst dispersed in pollutant solution in the dark for 15min. Many conditions such as concentration of catalyst and pollutant, time of irradiation and different pH values was studying to get best result.
Result and Discussion
Reaction Kinetic
α-Fe2O3 nanoparticles prepared using photolysis method. During this method, the irradiation of UV play as a reduce reagent where it works on reduction of ferric to ferrous ions and with the presence of the hydroxide ions in the solution, the remains ions of ferric combine with hydroxide to produce FeOOH while, the ferrous ions combine with hydroxide ions to get Fe(OH)2. Then, the FeOOH and Fe(OH)2 were burned to product brown nanoparticles of α-Fe2O3 as shown in below mechanism:
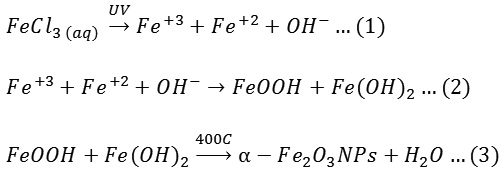
In the mechanism, hydrogen peroxide acted on fuctionalization of MWCNTS and these functional groups work as a site to adsorb the nanoparticles results of Vander wall forces or chemical interaction.
Analysis of XRD
XRD technique was used to investigate the structure of α-Fe2O3 and α-Fe2O3/MWCTS and the size of particles prepared. The XRD spectrum of α-Fe2O3 and α-Fe2O3/MWCTS was shown in figure 2. The characteristic peak of carbon MWCTS with small intensity appeared a clearly at 26.24 in spectrum of MWCTS and α-Fe2O3/MWCTS and the results is in agreement with (JCPDS no. 41-1487). When comparing the spectrum of MWCNTS and α-Fe2O3/MWCTS, any change wasn’t shown in the position of peaks just the peak of MWCNTS and this indicate incorporated the MWCNTS molecules in the lattice of nanocomposite.
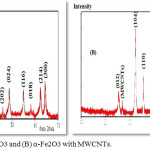 |
Figure 2: XRD of (A) α-Fe2O3 and (B) α-Fe2O3 with MWCNTs. |
Analysis of SEM
The morphology of samples prepared was studied and showed using scanning electron microscope (SEM). The morphology of α-Fe2O3 and α-Fe2O3/MWCNTS was shown in figures 2 (A) and 2 (B). The figure 2 (A) obtained prepare nano ferric oxide in rod shape with random distribution and it can be noted the particles fabricated are cylindrical shape while, the figure 2 (B) shows the presence of MWCNTS around or between the rod particle prepared and this indicate the conform of the preparation of α-Fe2O3/ MWCNTS nanocomposite. The ratio of nanocomposite elements was investigated by the EDS spectrum and it shows the high purity of the product as shown in figure 2 (C).
 |
Figure 3: SEM of (A) pure α-Fe2O3, (B) α-Fe2O3-MWCNTs and (C) EDS of α-Fe2O3-MWCNTs. |
Photodegradation of 4-chlorophenol by α-Fe2O3/MWCNTS
To investigate the photocatalyic activity of heterogeneous catalyst prepared, degradation of 4-nitrophenol solution in presence catalyst fabricated under UV irradiation was observed. In general, the results showed high efficiency for photocatalyic degradation of 4-chlorophenol. The degradation of pollutant followed the following mechanism17-19:
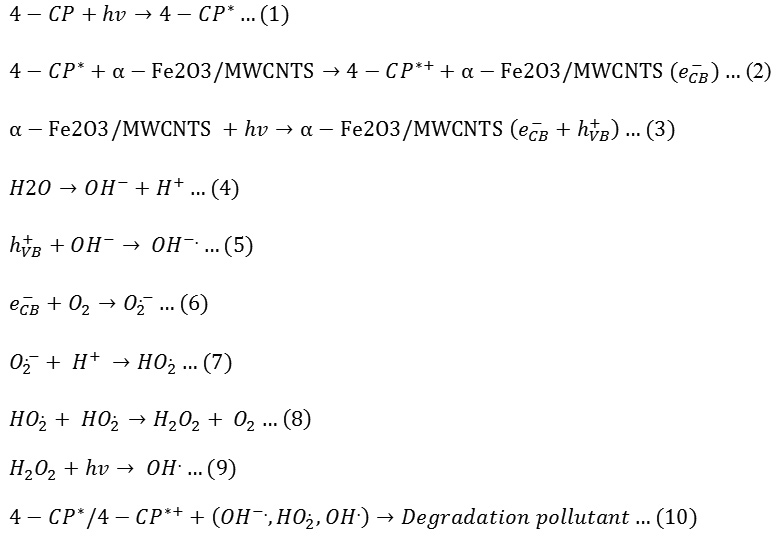
 |
Graph 1Click here to view graph |
The degradation mechanism proposes breaking the bond between C-Cl and the product which in turn react with oxygen to form 1,4-benzoquinone as shown in (Eq.11) and the results from GC-mass prove it as shown in figure 4. After irradiation, the spectra of GC-mass shows peak equal to 108g/mol assigned to 1,4-benzoquinone.
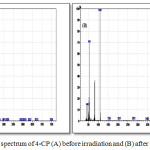 |
Figure 4: GC/mass spectrum of 4-CP (A) before irradiation and (B) after irradiation. |
Effect of pH
pH considered one of important factors that can be effected on degradation of pollutant due its effect on the rate of generating hydroxide free radicals and the charge of catalyst surface. To investigate the effect of it, the range from 3-10 of pH was studied. The results show that the best efficiency of degradation at 3 as shown in figure 5. At basic value, the repulsion force between catalyst and phenolate ions play the main role in decrease the efficiency while, in acidic medium the catalyst has a positive charge and it’s become a favorite for an adsorption of ions on the surface.20,21 on the other side, the ferric ions in oxide were reduced to ferrous ions in acidic medium and adsorbed on (001) surface of α-Fe2O3 and this cause the Fenton system. The charge of catalyst and the Fenton system can increase the efficiency of degradation.22
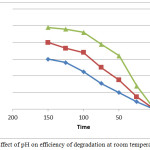 |
Figure 5: Effect of pH on efficiency of degradation at room temperature (2°C). Click here to view figure |
Effect of Catalyst Concentration
The photodegradtion efficiency of 4-CP was studied verse the a amount of catalyst used as shown in figure 6. The amount of catalyst ranged 0.1 – 0.5 g/L. The results appear the efficiency increase with the increase concentration and then decrease because with the increase it, the active site23 is increasing and the generation of hydroxyl free radicals increase that react with pollutants and destroyed it. Otherwise, the efficiency decreases with increasing the catalyst load due to the agglomeration of nanoparticles and this cause decrease of OH. that react with the pollutant.24-26
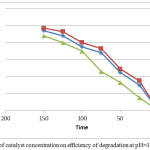 |
Figure 6: Effect of catalyst concentration on efficiency of degradation at pH=3 and temp =2°C. |
Effect of Initial Concentration of Pollutant
The effect of initial concentration of 4-CP on photodegradation rate was investigated in the range 20-80ppm. The results were illustrated in figure 7. The photodegradation yield depends on the reaction that occur on the surface of catalyst through reaction between OH free radicals and 4-CP. The results appeared the photodegradation efficiency decrease with increasing the concentration of 4-CP and this may be back to saturate of active sites of catalyst and cause to decrease the generate of hydroxyl free radicals27-28 as well as adsorbed the important amount of UV by pollutant instead of the catalyst.
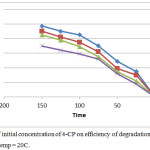 |
Figure 7: Effect of initial concentration of 4-CP on efficiency of degradation at pH=3, 0.1g catalyst and temp = 2°C. |
Conclusion
Alpha phase iron oxide with nanoparticles was can be prepared using the low temperature photolysis method and doping it with MWCNTS utilizing sonication method. UV irradiation play main role in the reduction ferric to ferrous ions and product geothite compound that react with ferrous hydroxide to get hematite nanoparticles. The results from XRD proved don’t enter the MWCNTS inside the crystal lattice of α-Fe2O3. The attack of hydroxyl free radicals on 4-CP destroyed the C-Cl bond and converted it to 1,4-benzoquinone.
Acknowledgements
The authors would like to acknowledge their department storage to supply the chemical materials.
References
- Xu, X.; Liu, Z.; Zhang, X.; Duan, S.; Xu, S.; Zhou, C. Electrochim. Acta. 2011, 58, 142–149.
CrossRef - Wu, J.; Wang, X.; Kang, H.; Zhang, J.; Yang, C. Int. J. Environ. Stud. 2014, 71, 534–545.
CrossRef - Peretz, S.; Cinteza, O. Eng. Asp. 2008, 319, 165–172.
CrossRef - Mishra, K.P.; Gogate, P.R. Ultrason. Sonochem. 2011, 18, 739–744.
CrossRef - Gao,Y.; Wang,Y.; Zhang, H. Appl. Catal. B: Environ. 2015, 178, 29–36.
CrossRef - Huang, Z.; Wu, P.; Lu,Y.; Wang, X.; Zhu, N.; Dang, Z. J. Hazard. Mater. 2013, 246–247, 70–78.
CrossRef - Nezamzadeh-Ejhieh, A.; Amiri, M. Powder Technol. 2013, 235, 279–288.
CrossRef - Phanikrishna Sharma, M.V.; Durga Kumari, V.; Subrahmanyam, M. Chemosphere. 2008, 73, 1562–1569.
CrossRef - Elmolla, E.S.; Chaudhuri, M. Desalination. 2010, 252, 46-52.
CrossRef - Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. J. Environ. Manage. 2011, 92, 311–330.
CrossRef - Gar Alalm, M.; Tawfik, A.; Ookawara, S. J. Water Process Eng. 2015, 8, 55–63.
CrossRef - Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. J. Phys. Chem. Lett. 2014, 5, 2543–2554.
CrossRef - Zaid, H.M.; Nuha, F.A.; Aklas, A.A. Asian J. Chem. 2018, 30, 223-225.
- Zaid, H.M. J. Chem Pharm Res. 2017, 9, 29-33.
CrossRef - Zaid H.M. DJPS. 2018, 14, 136-146.
CrossRef - Rawnaq, B. J.; Zaid, H. M.; Farah K. A. Entomol. Appl. Sci. Lett. 2018, 5 ,91-100.
- Sundaramurthy, J.; Suresh Kumar, P.; Kalaivani, M.; Thavasi,V.; Mhaisalkar,S.G.; Ramakrishna, S. RSC Adv.2012, 2, 8201–8208.
CrossRef - Shen,Y.; Zhao, Q.; Li, X.; Hou,Y.; Chen, G. Col. Surf. A: Physicochem. Eng. Asp. 2012, 403, 35–40.
CrossRef - Babuponnusami, A.; Muthukumar, K. J. Environ. Chem. Eng. 2014, 2, 557–572.
CrossRef - Carp, O.; Huisman, C.L.; Reller, A. Prog. Solid State Chem. 2004, 32, 33–177.
CrossRef - Pouretedal, H. R.; Motamedi, H.; Amiri, A. Desalin. Wat. Treat. 2012, 44, 92–99.
CrossRef - Huang, X.; Hou, X.; Zhao, J.; Zhang, L. Appl. Catal. B. 2016, 181, 127–137.
CrossRef - Sun, S. P.; Zeng, X.; Li, C.; Lemley, A.T. Chem. Eng. J. 2014, 244, 44-49.
CrossRef - Nezamzadeh-Ejhieh, A.; Shahriari, E. J. Ind. Eng. Chem. 2014, 20, 2719–2726.
CrossRef - Bahrami, M.; Nezamzadeh-Ejhieh, A. Mater. Sci. Semicond. Process. 2014, 27, 833–840.
CrossRef - Amiri, M.; Nezamzadeh-Ejhieh, A. Mater. Sci. Semicond. Process. 2015, 31, 501–508.
CrossRef - Assi, N.; Mohammadi, A.; Manuchehri, Q. S.; Walker, R. B. Desalin. Wat. Treat. 2015, 54, 1939–1948.
CrossRef - San, N.; Hatipog˘lu, A.; Kocturk, G., C¸ inar Z. J. Photochem. Photobiol. A: Chem. 2002, 146, 189–197.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









