Heterogeneous Zeolite-Based Catalyst for Esterification of α-Pinene to α-Terpinyl Acetate
Nanik Wijayati1,2, Ella Kusumastuti1, Dante Alighiri*1,2 , Baiti Rohmawati1 and Retno Ariadi Lusiana3
, Baiti Rohmawati1 and Retno Ariadi Lusiana3
1Essential Oil Study Center, Faculty of Mathematics and Natural Sciences, Universitas Negeri Semarang, Indonesia.
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Semarang, Indonesia.
3Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Diponegoro, Indonesia.
Corresponding Author E-mail: dante_alighiri@mail.unnes.ac.id
DOI : http://dx.doi.org/10.13005/ojc/350150
Article Received on : 01-11-2018
Article Accepted on : 23-01-2019
Article Published : 18 Feb 2019
The purpose of this study is to determine the most effective type of heterogeneous catalyst such as natural zeolite (ZA), Zr-natural zeolite (Zr/ZA) and zeolite Y (H/ZY) in esterification of α-pinene. α-terpinyl acetate was successfully synthesized from α-pinene and acetic anhydride by their heterogeneous catalysts. The esterification reaction was carried out with reaction time, temperature and zeolite catalysts. The most effective catalysts used in the synthesis of α-terpinyl acetate is catalyst H/ZY with the yield is 52.83% at 40°C for the time 4 hours with a selectivity of 61.38%. The results showed that the effective separation of catalyst could contribute to developing a new strategy for the synthesis of α-terpinyl acetate.
KEYWORDS:α-pinene; Esterification; Terpinyl Acetate; Zeolite
Download this article as:| Copy the following to cite this article: Wijayati N, Kusumastuti E, Alighiri D, Rohmawati B, Lusiana R. A. Heterogeneous Zeolite-Based Catalyst for Esterification of α-Pinene to α-Terpinyl Acetate. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Wijayati N, Kusumastuti E, Alighiri D, Rohmawati B, Lusiana R. A. Heterogeneous Zeolite-Based Catalyst for Esterification of α-Pinene to α-Terpinyl Acetate. Orient J Chem 2019;35(1). Available from: https://bit.ly/2GwQTVm |
Introduction
Terpinyl acetate is important to fine chemical and has been used in the fields of flavor, fragrance, food, medicine, cosmetics, organic synthesis and pharmaceutical industries.1-4 Terpinyl acetate synthesis can be done by an esterification reaction α-pinene with acid catalyst.5-7 Usually, it is prepared industrially in two stages: α-pinene is treated with liquid mineral acid and the resulting α-terpineol is then esterified with acetic anhydride and a catalyst.8-11
Esterification reaction of α-pinene has been carried out using homogeneous as well as heterogeneous catalysts. Heterogeneous catalysts have been considered as an alternative catalyst.12 They are metal oxides, zeolites, and active metals.13 The mechanism of the esterification catalyzed by heterogeneous catalysts is still debated.14,16 We have also proposed a similar intermediate in the esterification reaction using natural zeolite.
Several studies on the terpinyl acetate synthesis by α-pinene esterification reaction has been conducted by several previous researchers who have already converted α-pinene to terpinyl acetate with catalyst zeolite H-beta and it turns out the results is a concentration of terpinyl acetate by 29%.8 Li et.al. (2013) have done a terpinyl acetate synthesis with catalyst from ionic solutions with results of terpinyl acetate concentration by 30.8%,6 Terpinyl acetate is synthesized with lipase catalyst.17-19 The terpinyl acetate synthesis with this catalyst results terpinyl acetate as much as 40.3%.5
In this study, esterification of α-pinene to α-terpinyl acetate by using a heterogeneous catalyst such as natural zeolite (H/ZA), Zr-natural zeolite (Zr/ZA) and zeolite Y (H/ZY) were done to determine the most effective type of catalyst.
Experimental Section
The materials used in this study is turpentine oil from central Java, natural zeolite from Malang, and zeolite Y from Sigma Aldrich. Other chemicals such as sodium sulfate anhydrous (Na2SO4), zirconum (IV) chloride (ZrCl4), and acetic anhydride were purchased from Merck. All solid materials were directly used after drying without further purification.
Natural zeolite is soaked with 1% hydrofluoric acid (HF) solution for 30 minutes, then washed with aquademin, dried in an oven at 120°C for 3 hours. Natural zeolite is then soaked with hydrochloric acid (HCl) for 30 minutes at 50°C while stirring with a magnetic stirrer. The zeolite is washed with aquademin until the chloride ion (Cl–) disappears and then soaked with 1 N ammonium chloride (NH4Cl) solution. Natural zeolite is impregnated with 10% (w/w) of zirconium (Zr) metal. Zr/ZA and Y Zeolite catalysts were calcined at 500°C for 4 hours.
Characterization of the catalysts includes crystallinity analysis is observed by X-Ray Diffraction (XRD Shimadzu 6000), surface morphology by Scanning Electron Microscopy (SEM Phenom), acidity by gravimetric method and tested with Front Transmittance Infra Red (FTIR Perkin Elmer Version 10.4.00).
α-terpinyl acetate synthesis was carried out in a batch reactor with a magnetic stirrer. First, the 1 g of α-pinene, 10 mL acetic anhydride, 10 mL dichloromethane, and 5 mL of distilled water were introduced in the reactor, followed by the 0.5 g of catalysts. The reaction mixture was continuously stirred during the reaction using a magnetic stirrer at a temperature of 40°C. To optimize α-terpinyl acetate formation, experiments were conducted to study factors such as reaction time and type of catalyst. The reaction mixture is then separated from the catalyst with a centrifuge for 10 minutes and speed of 350 rpm. The reaction products were analyzed by Gas chromatography–mass spectrometry (GC-MS).
Result and Discussion
Preparation of Zr/ZA catalysts made by impregnating Zr metal. Zr metals are impregnated in ionic form, Zr4+cation that exchange with the cation H+in H/ZA. Activation of zeolite Y is done by calcination at a temperature 550°C for 4 h. Activation is aimed to enable the acid sites on the zeolite, remove organic substances, and eliminate gas products are still entrapped in the zeolite.
Characterization of catalyst crystallinity is performed by using XRD. Diffractogram of the catalyst H/ZA, Zr/ZA, and H/ZY is presented in Fig. 1.
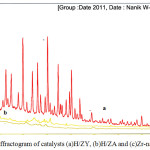 |
Figure 1: Diffractogram of catalysts (a)H/ZY, (b)H/ZA and (c)Zr-natural zeolite. |
Based on XRD analysis of H/ZY, H/ZA, and Zr/ZA, their angle position of diffraction (2q) and their distance between the field will describe the types of crystals. The sharp intensity on area 2q = 9.77°C, 21.45°C, 25.63°C, 27.49°C indicates the mordenite characteristic.20 The peak at 2q = 9.84°C, 21.34°C, 25.87°C, 27.21°C in natural zeolite sample from Malang indicated mordenite group in dominant phase.
The total acid sites based on data in Table 1. The samples H/ZY has the highest total acidity. In Zr/ZA catalyst has a higher acidity than H/ZA. This is due to the developing of zirconium metal in the catalyst. Zirconium has d orbitals that are not fully filled so can effectively accepts an electron pair from a base adsorbate. Donation amount of acid site of a zirconium metal is Lewis acid sites.21 The H/ZY catalyst has a higher acidity value than the H/ZA and Zr/ZA catalysts so that the H/ZY catalyst is more effective in the esterification reaction.
Table 1: The total acidity of Zeolite H/ZY, H/ZA, and Zr/ZA.
| Material | Total acidity (mmol/g) |
| H/ZA | 0.81 |
| Zr/ZA | 5.70 |
| H/ZY | 8.87 |
Acidity for each adsorbent and catalyst depends on the nature of the adsorbent and the catalyst surface, which can be a Lewis acid or Bronsted acid. To be able to distinguish the type of Bronsted acid sites (1640 cm-1) and Lewis acid sites (1400 cm-1) in the zeolite, it can be seen from Fig 2. The presence of the Lewis acid can appear at the top of the range of 1450 cm-1, whereas the Bronsted acid appears at the top of the range 1550 to 1640 cm-1. A weak peak at 788 cm-1 appears in the spectrum of the modified zeolite.10,22
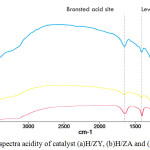 |
Figure 2: FT-IR spectra acidity of catalyst (a)H/ZY, (b)H/ZA and (c)Zr/ZA. |
Analysis of the morphology of the catalyst surface was performed using SEM instrument to observe microscopically catalyst H/ZA, Zr/ZA, and H/ZY (Fig. 3). The images specify that the zeolite surface is coated by Zr4+. In catalyst H/ZA has irregular morphology, such as the hollow porous rocks. Based on the results of XRD, natural zeolite Malang has the characteristics of mordenite crystals shaped like needles, but the results are not visible needle shape, while the Zr-zeolite catalyst morphology seems natural form mordenite crystals resemble needles. Based on the results of SEM magnification 10000x known that catalyst H/ZY have a uniform morphology in the form of granules resembling an irregular cube. Based on the results of SEM magnification 10000 x known that the form of the catalyst H/ZY is in the form of granules resembling an irregular cube.
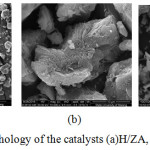 |
Figure 3: The morphology of the catalysts (a)H/ZA, (b), Zr/ZA(c)H/ZY. |
The greatest concentration of product α-terpinyl acetate in esterification reaction performed from the reaction by using a catalyst H/ZY. The H/ZY catalyst has the highest acidity values than ZA and Zr/ZA catalyst so that the catalyst H/ZY more effectively carry out the esterification reaction. In catalyst Zr/ZA has an acidity value higher than the ZA catalyst, but on the outcome of the esterification reaction α-pinene level of α-terpinyl acetate products that produced is lower, it could be due to the Zr metal is too large so that the closed pore zeolite and finally inhibit the action of catalysts in α-terpinyl acetate formation.
Table 2: Concentration and selectivity of α-terpinyl acetate.
| Catalysts | Conversion (%) | Selectivity (%) |
| H/ZY | 52.83 | 61.38 |
| H/ZA | 28.77 | 30.41 |
| Zr/ZA | 23.53 | 24.64 |
The α-pinene esterification with temperature variations of 30, 40 and 60°C with a reaction time of 3 hours and the ratio of pinene acetic anhydride compound (1:15) yielded α-terpenyl acetate with 0.18, 21.40, and 14.72% with the conversion of 4.99, 74.13 and 52.44. The effect of reaction temperature on α-pinene esterification is shown in Table 2. When the temperature was low, the yield of α-terpinyl acetate was low. As the temperature was increased, the yield of α-terpinyl acetate increased accordingly. When the temperature was high, the yield of α-terpinyl acetate tended to decrease. The results showed the yield of α-terpinyl acetate first increased and then decreased with increasing temperature.
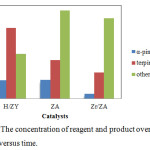 |
Figure 4: The concentration of reagent and product over catalysts versus time. |
Fig. 4 shows the effect of various heterogeneous catalysts on the reaction, including all minor products. The reaction time was 4 hours, α-terpinyl acetate reached its highest value of 52.83%.
Table 3: The effect of reaction temperature on α-pinene esterification reaction.
| Temp (ºC) | Conversion (%) | Yield (%) | Selectivity of α-terpinyl acetate | |||||
| Fencyl alcohol | Borneol | α-terpineol | Fencyl acetate | Bornil acetate | α-terpinyl acetate | |||
| 30 | 4.99 | 0.15 | 0.14 | 0.74 | 0.33 | 0.94 | 0.18 | 3.68 |
| 40 | 74.13 | 1.67 | 0.44 | 43.31 | 3.76 | 1.02 | 21.4 | 28.87 |
| 60 | 52.44 | 0.35 | 23.62 | 1.21 | 1.65 | 1.65 | 14.72 | 28.08 |
Reaction conditions: 0.5 g of pinene, 10 mL of acetic anhydride, 5 mL H2O, 10 mL dichloromethane, reaction time of 3 hours.
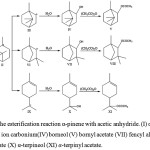 |
Figure 5: The esterification reaction α-pinene with acetic anhydride. (I) α-pinene (II, III, VI, IX) ion carbonium(IV) borneol (V) bornyl acetate (VII) fencyl alcohol (VIII) fencyl acetate (X) α-terpineol (XI) α-terpinyl acetate. |
As shown in Fig 5, once the carbonium ion is produced by protonation of α-pinene, there exists a competition between ring enlarging (II, III, IV) and ring-opening reactions (VI). In the ring-opening reaction, the esterification product is α-terpinyl acetate. The esterification reaction of α-pinene as “in situ” in a reaction that occurs through hydration reactions prior to the addition of distilled water to form α-terpineol then α-terpineol with acetate anhydride to form α-terpinyl acetate (XI) and by-products such as bornyl acetate (V) and fenchyl acetate (VIII). A similar behavior was observed by Liu et al. (2013),6 and Lu et al. (2008).9
Conclusion
Synthesis of α-terpinyl acetate can be done by an esterification reaction α-pinene as “in situ” with the heterogeneous zeolite-based catalysts. The most effective catalysts used in the synthesis of α-terpinyl acetate is catalyst H/ZY with the largest concentration product yield is 52.83% at 4 h with a selectivity of 61.38%.
Acknowledgments
The authors would like to thank Ditjen Penguatan Riset dan Pengembangan, Kemenristekdikti, Republic Indonesia for to support.
References
- Lee, Y.; Park, H.-G.; Kim, V.; Cho, M.-A.; Kim, H.; Ho,T.-H.; Cho, K.S.; Lee,I.-S.; Kim, D. Chemico–Biological Interactions, 2018, 289, 90–97.
CrossRef - Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Annu. Rev. Plant Biol.,2014, 65,225–257.
CrossRef - Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Greim, H.; Hanifin, J. M.; Rogers, A. E.; Saurat, J. H.; Sipes, I.G.; Tagami, H. A. Food Chem. Toxicol.2008, 46, S1.
CrossRef - Catrinescu, C.; Fernandes, C.; Castilho, P.; Breen, C. Applied Catalysis A: General, 2014, 489, 171–179.
CrossRef - Liaw, E.-T.; K.-J. Liu. Bioresource Technology, 2010, 101, 3320–3324.
CrossRef - Li, L.; Liu,S.; Shi,Y.; Yu,S.; Xie,C.; Qi, C. Res Chem Intermed, 2013, 39, 2095–2105.
CrossRef - Gusevskaya, E. V. Chem Cat Chem, 2014, 6, 1506–1515.
CrossRef - Gainsford, G. J.; Hosie, C. F.; Weston, R. J. Applied Catalysis A., 2001, 209, 269–277.
CrossRef - Liu, S.; Xie,C.; Yu,S.; Liu,F.; Ji, K.Catal. Commun, 2008, 9, 1634–1638.
CrossRef - Wijayati, N.; Handayani, T.; Supartono. Asian Journal of Chemistry. 2017, 29, 1705–1708.
CrossRef - Liu, S. W.; Yu, S. T.; Liu, F. S.; Xie, C. X.; Li, L.;Ji, K. H. Journal of Molecular Catalysis A: Chemical,2008, 279, 177–181.
CrossRef - Atalay, B.; Gündüz, G. Chemical Engineering Journal, 2011, 168, 1311–1318.
CrossRef - Zilfa; Rahmayeni; Stiadi, Y.; Adril. Oriental Journal of Chemistry,2018, 34, 887-893.
CrossRef - Wijayati, N.; Pranowo, H. D.; Jumina; Triyono. Indo. J. Chem,2013, 13,59–65.
CrossRef - Mitran, G.; Pavel, O. D. Reac. Kinet. Mech. Cat., 2015,114, 197–209.
CrossRef - Kirumakki, S.R.; Nagaraju, N.; Komandur, V.R.; Chary. Applied Catalysis A. General, 2006, 299, 185–192.
CrossRef - Rosa, B. H.; Silva, G. S.; Conceição, G. J. A.; Carvalho, R. A.; Aguiar-Oliveira, E.; Maldonado, R. R.; Kamimura, E.S. Biocatalysis and Agricultural Biotechnology,2017, 12, 90–95.
CrossRef - Badgujar, K.C.; Bhalchandra, M.Process Biochem., 2014, 49, 1304–1313.
CrossRef - Gupta, A.; Dhakate, S.R.; Pahwa, M.; Sinha, S.; Chand, S.; Mathuer, R.B. Process Biochem., 2013,48, 124–132.
CrossRef - Treacy, M.M.J. & J.B. Higgins. Collection of Simulated XRD Powder Patterns for Zeolites. Amsterdam: Elsevier, 2001.
- Comelli, N.; Ponzi,E.; Ponzi, M. Chemical Engineering Journal, 2006,117, 93–99.
CrossRef - Ryczkowski, J.Catalysis Today,2001, 68, 263–381.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









