Faloak (Sterculia Quadrifida R.Br) Stem Bark Extract Inhibits Hepatitis C Virus JFH1
Muhajirin Dean*1 , Retno Handajani2 and Junaidi Khotib3
, Retno Handajani2 and Junaidi Khotib3
1Department Pharmacy, Prof. Dr. W.Z. Johannes Hospital, Jalan Dr. Moch Hatta No. 19, Kupang City 85112, East Nusa Tenggara, Indonesia.
2Department Biochemistry, Medical Faculty, Airlangga University, Jalan Mayjen Prof. Dr. Moestopo No. 47, Surabaya 60131, East Java, Indonesia.
3Department of Clinical Pharmacy, Faculty of Pharmacy, Airlangga University, Jalan Dharmawangsa No. 4-6, Surabaya 60286, East Java, Indonesia.
Correspondent Author E-mail: muhajirindean@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/350155
Article Received on : 29-11-2018
Article Accepted on : 07-01-2019
Article Published : 07 Feb 2019
The discovery of antiviral drugs is developing new alternative medication and economics prices. One of the natural resources for alternative medicine is the water extract of faloak stem bark which is a material used by Kupang Community as an antiviral drug. The HCV JFH1 lifecycle inhibition is expressed in IC50 (inhibitory concentration 50%) and the toxicity of water extract of faloak stem bark in hepatocyte cell line Huh7it is expressed in CC50 (cytotoxicity concentration 50%). Determining the results obtained using Microsoft Office® Excell 2013®. The water extract of faloak stem bark has an inhibition of HCV genotype 2a strain JFH1 with the IC50 11.57μg/mL and the toxicity water extract of faloak stem bark in the Huh7it cell line hepatocyte with the CC50 >1000μg/mL. Mode of action activities from water extract of faloak stem bark can be inhibited all step HCV life cycle. The first step is entry step has the inhibition 93.97%, post-entry step has the inhibition 96.75%, and combination step (entry and post-entry step) has the inhibition was 100%. The active compound in the water extract of faloak stem bark was epicatechin 875mg/kg. The water extract of faloak stem bark can be a candidate for antivirals against HCV JFH1.
KEYWORDS:Faloak; Hepatitis C Virus; Sterculia Quadrifida R.Br
Download this article as:| Copy the following to cite this article: Dean M, Handajani R, Khotib J. Faloak (Sterculia Quadrifida R.Br) Stem Bark Extract Inhibits Hepatitis C Virus JFH1. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Dean M, Handajani R, Khotib J. Faloak (Sterculia Quadrifida R.Br) Stem Bark Extract Inhibits Hepatitis C Virus JFH1. Orient J Chem 2019;35(1). Available from: https://bit.ly/2URjNTl |
Introduction
Hepatitis C virus infection is a global problem and needed drugs can inhibit HCV infection. There is now a combination with pegylated interferon alfa (IFN-α) and ribavirin.1 However until now the fulfillment of the drug needs has not been met well throughout the region of Indonesia, it takes an alternative treatment to help HCV infection. One medicinal plant that can be used as an alternative treatment for hepatitis is faloak. The part of the faloak plant commonly used as medicine is the stem bark.2 Tests have been carried out on the faloak bark and flavonoid compounds have been found. Flavonoid compounds can inhibit the hepatitis C virus in cell culture4 so that faloak plants can be tested to obtain active content and the effect of inhibiting the hepatitis c virus.
Materials and Methods
Materials
The stem bark of faloak is obtained from Kupang City of East Nusa Tenggara Province. The only stemming by peeling is taken from the main stem. Weighed fresh samples, cut into small pieces and dried by air-dried until dried and then fertilized and weighed dry samples. Extraction is performed using a water solvent. The obtained viscous extract is dried using a freeze dryer until the extract is dry and weighed.
Methods
Preparation and Sample Extraction
Determinants plants in UPT BKT Purwodadi Garden, East Java, Indonesia. Extraction was extracted for LC-MS/MS test to identify epicatechin compounds. A regular concentration series of (-)-epicatechin >98% (Sigma-Aldrich) was dissolved using 0.5 gram of water extract from new bark to make a standard curve. A weigh of 0.5 grams of homogeneous water extracts of bark dissolved in methanol and vortex until. Then the extract solution (0.1 mL) was added to 0.9 mL of methanol 50%, and then it was vortexed. The calculation of epicatechin level using dilution factor 1000, then the sample is injected into LC-MS/ MS (AB-Sciex 4000) instrument. The result is a chromatogram and contains a peak epicatechin compound from the Analyst Instrument Control and Data Processing Software AB Sciex which is software in LC-MS/MS (AB-Sciex 4000) instrument.
Cells and Viruses
Analysis of anti-HCV Activities
The anti-HCV test was carried out following the previous test.5,6 The sampels was dissolved in dimethyl sulfoxide (DMSO) to obtain stock solutions at a concentration of 100mg/ml, stored at -20°C until it was used. Huh7it cells were seeded in 48-well plates (5×104 cells/well). A fixed amount of infection (FFU)/cell, was mixed with serial dilutions of the extracts (100, 50, 25, 12.5, 6.25 and 3.125μg/ml) and inoculated to the Huh7it cells. After two hours incubation, the cells were washed with Dulbecco’s modified Eagle’s medium to remove the residual virus and further incubated in the Dulbecco’s modified Eagle’s medium containing the same concentrations of test samples as those during virus inoculation.
Stainning
Cell staining was carried out following the previous test.5 The Huh7it cells were prepared medium in 96-well plate (2.3 x 104 cell/mL) incubation 24 hours. The stored supernatant was thawed first and serially diluted in medium with 2% FBS, added into the Huh7it cells, incubation 2 hours at 37°C with 5% CO2. Then, remove supernatant and overlaid with methylcellulose 0.5%, incubation at 37°C with 5% CO2 for two days. After two-day infection cells, in each well were fixated using 200μL 10% formaldehyde per well and incubated for 15 minutes. After the formaldehyde was removed, the cells were washed three times with 200μL of Phosphate Buffered Saline (PBS) with a period of 5 minutes in between. After the PBS was removed, 100μL of Triton-X 0.5% was added to each well. Afterward, incubation was done for 10 minutes. The Triton-X was then removed from all wells. Next, the wells were rinsed with PBS three times with 200μL of PBS with a period of 5 minutes in between. Then, 45μL of 1/200 anti-HCV from human serum was added. Incubation for 1 hour was then performed. After removing the antibody, each well was rinsed with PBS. After that, the second antibody, which was 45μl of 1/300 human anti-horseradish peroxidase (HRP) (Sigma-Aldrich), was added. It was incubated, removed, and rinsed again with the same method as before. Next, 100μL of 3, 3-diaminobenzidine (DAB) (Thermo Scientific, USA) was added to each well as staining step. Then, it was incubated for 15 minutes. Finally, the virus focus was observed under light microscope and calculated with Katikati® program as cells counter.
Cytotoxic Assay
The cytotoxic test followed the previous test.5 The cell viability of the samples was assessed by (3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide) (MTT) (Sigma-Aldrich) assay. Huh7it cells (2.3×104 cells/well) in 96 well plates were treated with serial dilution of the samples (1000μg, 800μg, 600μg, 400μg, 200μg. 100μg, 50μg, 25μg12.5μg). The condition of the cells was observed after 46 hours incubation, and the toxicity was checked under a microscope. The medium was removed from 96 well plates and then MTT 10% 150 μl/well was put by multichannel pipette and incubated for 4 hours at 37°C. MTT solution was removed from 96 well plates and 100µl/well DMSO 100% was then put for dissolve. Absorbance was checked at 560 nm and 750 nm, shaker 0.5 min before reading absorbance. The MTT reagent is absorbed by the cells and converted by reduction reaction to formazan by mitochondrial dehydrogenases. Percent cell viability compared to the control was calculated for each dilution of the samples and CC50 were determined.
Mode of Action Assay
The mode of action test is carried out by following the previous test.5,7 These experiments were performed to assess the way of action of the samples. It has two sets of tests; the first is entry step, the mixture of HCV and sample was inoculated to the cells. After 2 hours, the residual virus and the sample were removed, and cells added to the medium without samples for 46 hours. The second step is a post entry step, HCV was inoculated to the cells in the absence of the sample. After 2 hours, the residual virus was removed, and the cell was added to the medium containing the sample for 46 hours. Positive control used a medium containing 0.1% DMSO.
Results
Taxonomically, faloak plants are included in the Genus Sterculia,8,9 so the initial assumption of faloak plants contains compounds that resemble plants in their genus. Plants that are one genus, namely Sterculia tragacantha, which has successfully isolated several flavonoid compounds, one of these compounds is epicatechin.10 On this basis, the search for epicatechin compounds was carried out qualitative and quantitative by using LC-MS / MS (AB-Sciex 4000).10 The test results using LC-MS/MS (AB-Sciex 4000) library software water extract faloak stem bark identified containing epicatechin at retention time (RT) and mass to charge ratio (m/z) shown in Figure 1.
 |
Figure 1: Chromatogram results of the identification of epicatechin identification compounds in the water extract of water extract faloak stem bark to determine RT using LC-MS/MS (AB-Sciex 4000). |
(A) From the figure at RT 3.90 shows a compound based on software library from LC-MS/MS (AB-Sciex 4000) so that this pick graph is an epicatechin compound. (B) The epicathechin compounds in the water extract faloak stem bark is shown at m/z 289.2 based on the software library LC-MS/MS (AB-Sciex 4000) with the molecular formula C15H15O61+.
After successfully identifying the epicatechin compounds in the water extract of faloak stem bark, the next step is to determine the level of epicatechin compounds. Determination of epicatechin compounds using LC-MS/MS (AB-Sciex 4000) and standard compounds of standard compounds (-)-epicatechin >98% (Sigma-Aldrich). The results of the determination of the levels of epicatechin compounds in the water extract of faloak stem bark shown in Figure 2.
 |
Figure 2: Chromatogram results of the identification of epicatechin identification compounds in the water extract of faloak stem bark to determine RT using LC-MS/MS (AB-Sciex 4000). |
(A) The epicatechin standard at RT 3.90. (B) The epicathechin compounds in the water extract of faloak stem bark is shown at RT 3.90.The calculation of epicatechin concentration obtained by using Analyst Instrument Control and Data Processing Software AB Sciex obtained by linear regression equation y=104 x+2.88 and R-value =0,9993. Total epicatechin found in a high concentration of 875mg/kg. These results are in accordance with the initial assumptions that mention faloak plants contain epicatechin compounds.
Anti-HCV Activities of Sterculia Quadrifida R.Br Stem Bark
The water extract of faloak stem bark at the concentration of 50 and 100μg/mL showed 100% inhibition to HCV JFH1 replication. Lower doses such as 25μg/mL, 12.5 μg/mL, 6.25μg/mL and 3.125 μg/mL showed the percentage of infectivity at the level of 95.39%, 67.50%, 29.775% and 8.31%, respectively. From the linear regression equation of the percent infectivity, IC50 of water extract of faloak stem bark 11.67μg/mL (Figure 3).
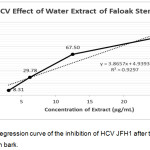 |
Figure 3: Linear Regression curve of the inhibition of HCV JFH1 after treated with water extract faloak stem bark. |
The data represent means ± SEM of data from two independent experiments.
Cytotoxic Assay
The cytotoxic test using MTT assay aims to calculate the viability of cells and then compared with the viability after treated with DMSO. The results showed that the treatment using water extract of faloak stem bark at the concentration of up to 100μg/mL was not toxic to the cells, while the cell viability decreased at treatment with 200μg/mL. From the linear regression equation of the percent viability, we found that the CC50 of water extract of faloak stem bark was >1000μg/mL (Figure 4).
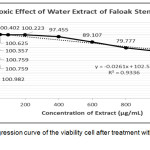 |
Figure 4: Linear regression curve of the viability cell after treatment with water extract of faloak stem bark. |
The data represent means ± SEM of data from two independent experiments.
After testing the effect of HCV JFH1 inhibition and Huh7it cell line cytotoxic test, it was continued by determining the selectivity index with the criteria >3.11 The selectivity index is determined by comparing CC50 and IC50.12 The results of the comparison are obtained with> 8.57, then the next test which is the time of the additional test can be done.
Mode of Action Anti-HCV
Test to determine the inhibition step of water extract of faloak stem bark the HCV JFH1 lifecycle using a concentration of 40μg/mL and obtained the test results at the entry step was 93.97% inhibition. The post-entry step was 96.75% inhibition. Combination of entry and the post-entry step was 100% inhibition. The results can be seen in Figure 5.
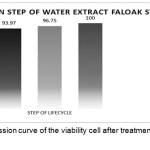 |
Figure 5: Linear regression curve of the viability cell after treatment with water extract of faloak stem bark. |
The data represent means ± SEM of data from three independent experiments. From the data, 100% inhibition was encountered during and post-inoculation step, the inhibition was 93.968% at the step during inoculation and the inhibition was 96.75% at the post inoculation step. Thus showing the water extract of faloak bark can inhibit at three step in the HCV JFH1 life cycle.
Discussion
Water extract of faloak stem bark has flavonoid content, namely epicatechin which is soluble in water.13 This result is similar to previous researchers, epicatechin compounds contained in the extract have the ability as anti-HCV14,15 and not toxic.16
The results of the inhibitory test of water extract of faloak stem bark were 11.67μg/mL, these results met the criteria of inhibitory extracts in vitro testing that was equal to ≤25μg/mL.11 The results of cytotoxic tests obtained results >1000μg/mL, these results indicate Cell viability at a concentration of 1000μg/L cell viability was 73.97% and this was according to the criteria of >50%.11 It also obtained a selectivity index of >8.57. From these data water extract faloak stem bark can be continued to the next test.
The test results of the step of inhibition in the HCV JFH1 life cycle obtained by the extract of faloak bark water can inhibit at the entry step. In this entry step the water extracts of faloak stem bark interacting with binding factors (GAGs and LDL-R), receptors (SR-BI, CD81, Occludin and Cloudin 1) and entry factors (EGFR, EphA2, TfR1 and NPC1L1)17,18,19 and VHC JFH1, which is expected to be able to inhibit the life cycle of JFH1 by inhibiting one of the above things by 93.968%. At the post-entry stage also water extract faloak stem bark can inhibit the life cycle of JFH1 HCV. At this stage the water extract faloak bark will interact internally, especially the non-structural parts that function in RNA replication such as NS3, NS3 / NS4A, NS4B, NS5A and NS5B.20 From the data, it is found that there are obstacles around 96.75%, so that it has obstacles in one or several non-structural proteins. This research can be continued for testing specific proteins in the JFH1 VHC infection process in Huh7it cell line hepatocyte culture in vitro.
Conclusion
Faloak plants for the first time identified and determined the content of epicatechin compounds. It can inhibit VHC JFH1 replication, is not toxic and can inhibit HCV JFH1 at the entry and post-entry step in the Huh7it hepatocyte cell line.
Acknowledgements
This work is supported by the Bumi Flores Group Company in Kupang City, Indonesia.
Conflict of Interest
There is no conflict of interest.
References
- Qian, X. J.; Zhu, Y.Z.; Zhao, P.; Qi, Z. T. Entry inhibitors: New advances in HCV treatment. Emerg Microbes Infect. 2016;5(October 2015):e3.
- Ranta, F.; Nawawi, D.; Pribadi, E.; Syafii, W. Aktivitas Anticendawan Zat Ekstraktif Faloak (Sterculia comosa Wallich). J Ilmu dan Teknol Kayu Trop. 2012;10(1):60–5.
- Ciesek, S.; von Hahn, T.; Colpitts, C. C.; Schang, L. M.; Friesland. M.; Steinmann, J., Manns, M. P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; Pietschmann, T.; Steinmann, E. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54(6):1947–55.
- Hafid, A. F.; Aoki-Utsubo, C.; Permanasari, A. A.; Adianti, M.; Tumewu, L.; Widyawaruyanti, A.; Wahyuningsih, S. P. A.; Wahyuni, T. S.; Lusida, M. I.; Soetjipto; Hotta, H. Antiviral activity of the dichloromethane extracts from Artocarpus heterophyllus leaves against hepatitis C virus. Asian Pac J Trop Biomed. 2017;7(7):633–9.
- Tumewu, L.; Apryani, E.; Santi, M.R.; Wahyuni, T. S.; Permanasari, A. A.; Adianti, M.; Aoki, C.; Widyawaruyanti, A.; Hafida, A. F.; Lusida, M. I.; Soetjipto; Hotta H. AntiHepatitis C Virus Activity of Alectryon serratus Leaves Extract. Procedia Chem . 2016;18(December):169–73.
- Hafid, A. F.; Permanasari, A. A.; Tumewu, L.; Adianti, M.; Aoki, C.; Widyawaruyanti, A.; Soetjipto; Lusida, M. I.; Hotta H. Activities of Ficus fistulosa Leave Extract and Fractions against Hepatitis C Virus. Procedia Chem. 2016;18(Mcls 2015):179–84.
- Rollando, R.; Alfanaar, R. Isolasi Senyawa Turunan Naptokuinon Dari Kulit Batang Faloak (Sterculia quadrifida R. BR) dan Uji Aktivitas Antikanker Pada Sel Kanker Payudara Jenis T47D. Indonesian E-Journal Appl Chem. 2017;5(1):12–7.
- Siswadi; Saragih, G. S.; Rainawati, H. Potential distribution and utilization of Faloak (Sterculia quadrifida R.Br 1844) on Timor Island, East Nusa Tenggara. In: Forest and Biodiversity. 2013.
- Oryzakeye, O.T.; Olugbade, T. A. Epicatechin and Procyanidin B2 in the Stem and Root bark of Sterculia tragacantha Lindl (Sterculiaceae). Med Chem (Los Angeles). 2014;04(02):334–7.
- Takeshita, M.; Ishida, Y. I.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Uto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from Blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J Biol Chem. 2009;284(32):21165–76.
- Saptawati, L.; Febrinasari, R. P.; Yudhani, R. D.; Faza, A. G.; Ummiyati, H. S.; Sudiro, T. M.; Dewi, B. E. In vitro study of eight Indonesian plants extracts as anti Dengue virus. Heal Sci J Indones. 2017;8(1):12–8.
- Aoki, C.; Hartati, S.; Santi, M. R.; Lydwina; Firdaus, R.; Hanafi, M.; Kardono, L. B. S.; Shimizu, Y.; Sudarmono, P.; Hotta, H. Isolation and identification of substances with anti-hepatitis c virus activities from kalanchoe pinnata. Int J Pharm Pharm Sci. 2014;6(2):211–5.
- Amin, A.; Wunas, J.; Anin, Y. M. Uji Aktivitas Antioksidan Ekstrak Etanol Klika Faloak (Sterculia quadrifida R.Br) Dengan Metode DPPH (2,2-diphenyl-1-picrylhydrazyl). J Fitofarmaka Indones. 2013.
- Chen, W. C.; Tseng, C. K.; Chen, B. H.; Lin, C. K.; Lee, J. C. Grape seed extract attenuates hepatitis C virus replication and virus-induced inflammation. Front Pharmacol. 2016;7(DEC):1–11.
- Siswadi; Rolando. Penelusuran Potensi Aktivitas Sitotoksik Fraksi Kulit Batang Tumbuhan Faloak (Sterculia quadrifida R.Br). e-Publikasi Fak Farm. 2016;
- Zhu, Y. Z.; Qian, X. J.; Zhao, P.; Qi, Z. T. How hepatitis C virus invades hepatocytes: The mystery of viral entry. World J Gastroenterol. 2014;20(13):3457–67.
- Qian, X. J.; Jin, Y. S.; Chen, H. S.; Xu, Q. Q.; Ren, H.; Zhu, S. Y.; Tang, H. L.; Wang, Y.; Zhao, P.; Qi, Z. T.; Zhu, Y. Z. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J Gen Virol. 2016;97(5):1134–44.
- Irshad, M.; Mankotia, D. S.; Irshad, K. An insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J Gastroenterol. 2013;19(44):7896–909.
- Calland, N.; Dubuisson, J.; Rouillé, Y.; Séron, K. Hepatitis C virus and natural compounds: A new antiviral approach? Viruses. 2012;4(10):2197–217.

This work is licensed under a Creative Commons Attribution 4.0 International License.









