Electrochemical Capacitive Performance of Zncl2 Activated Carbon Derived from Bamboo Bagasse in Aqueous and Organic Electrolyte
Sivagaami Sundari Gunasekaran1, Raghu Subashchandra Bose2 and Kalaivani Raman1
1Department of Chemistry, Vels Institute of Science, Technology and Advanced Studies (VISTAS), Chennai -117, India.
2Centre for Advanced Research and Development (CARD), Vels Institute of Science, Technology and Advanced Studies, Chennai -117, India.
Corresponding Author E-mail: rakvani@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/350136
Article Received on : 07-12-2018
Article Accepted on : 04-01-2019
Article Published : 25 Jan 2019
The present aims to explore the supercapacitor performance with the zinc catalysed activated carbon from biomass (bamboo bagasse) as the electrode material. The zinc-catalysed activated carbon is prepared by a novel combined technique of hydrothermal followed by chemical activation process. The prepared carbon was subjected to electrochemical characterization like cyclic voltammetry, galvanostatic charge-discharge, electrochemical impedance spectroscopy and cycling studies. The carbon activated in the presence of zinc chloride has the highest capacitance of 180 Fg-1 in aqueous medium and 153 Fg-1 in the non-aqueous medium.
KEYWORDS:Activated Carbon; Carbonization; Energy Storage Materials; Supercapacitor
Download this article as:| Copy the following to cite this article: Gunasekaran S. S, Bose R. S, Raman K. Electrochemical Capacitive Performance of Zncl2 Activated Carbon Derived from Bamboo Bagasse in Aqueous and Organic Electrolyte. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Gunasekaran S. S, Bose R. S, Raman K. Electrochemical Capacitive Performance of Zncl2 Activated Carbon Derived from Bamboo Bagasse in Aqueous and Organic Electrolyte. Orient J Chem 2019;35(1). Available from: https://bit.ly/2MVWnJA |
Introduction
There is an enhancement in the shortage of energy and there is a commitment to delve into authentic, sustainable and competent form of energy source without pollutions. Supercapacitors are regarded as the imperative type of energy storage and supply devices. Electrical double layer capacitors (EDLC) and Pseudocapacitor are the two main classifications of supercapacitor. The electrochemical performance is highly dependent on both the physical and chemical characteristic of the electrode material. As a result of being low cost and more availability, carbons, carbon nano tubes (CNT), graphene, activated carbons, biomass derived carbons are prepared as the electrode materials for the supercapacitor applications.1
Supercapacitors has many merits like longer time of life cycle which would be greater than 100,000 cycles, greater than 1500 Wkg-1 power density and upto 10 Whkg-1 energy density.2-4 From literature, many materials like electro-active polymers, transition metal-oxides are employed as the supercapacitor electrode material.5,6 Among different materials used, carbon materials played a vital role as the electrode material because of its low cost, satisfactory electronic conductivity, larger surface area and availability. The engaging point of the carbons used economically is the activated carbon.7 Here, the charge is gathered by the electrostatic charge separation of ions at the electrode-electrolyte interface which forms a double layer. Generally, most of the electrodes used in the supercapacitors have some amount of binder in it, like Teflon or polyvynilidene chloride, which creates uniformity in the electrode film, but however, they tend to get high electrical resistance which makes ultimate decrease in their capacitance. Hence, the alternative for this limitation is the used of binder-less electrode material. So, they can used without carbon additives, and they are easy for the handling.8-11
Bamboo based activated carbon (AC) electrode for supercapacitor application have been reported in the previous study with KOH and KOH+Fe3O4 as the activating agent and obtained 160 Fg-1 of specific capacitance in the aqueous medium.1
Carbonization of the precursor material (biomass) for the supercapacitor electrode leads to around 30% yield when it is done by habitual method. But, when it is done with the aid of chemical activating agents which can be dehydrating agents can enhance the percentage (%) of char yield and also it can influence on the thermal degradation and the generation of the porosity in the precursor material. Here, the biomass (precursor) undergoes contraction process during carbonization. This process of contraction is important when happens in the presence of chemical activating agents, as the activating agent incorporated into the interior parts of the precursor particles. As the activation temperature is increased, the contraction process decreases.12
The objective of this work is to present the electrochemical and supercapacitor performance of the bamboo derived carbon activated with the most commonly used zinc chloride (ZnCl2) and find out the dominant performer supercapacitor electrode. The relation between the surface characteristic, pore size and the capacitance is briefly discussed.13
Materials and Methods
ZnCl2 were purchased from Sigma Aldrich. Potassium hydroxide (KOH), iso-propyl alcohol (IPA), Teflon solution (60%), conducting carbon (ethylene black) were purchase from sigma Aldrich. The organic (tetraethylammonium tetrafluoroborate- Et4NBF4) electrolyte was bought from Top Machine Pvt. Ltd, China. All the chemicals that are used in this experiment are of analytical grade and were used without any further purification.
Characterization Techniques
X-ray Diffraction (XRD) spectroscopic study was carried out on a Bruker D8 Advances instrument using Cu-Κα (λ = 1.5406 A) radiation in the 2θ range from 5° to 80° with an acceleration voltage of 40 KV. The surface morphology and the elemental composition of graphene were investigated using (Scanning Electron Microscopy) Hitachi S-4700/JEOL JSM-6500F microscope at an accelerated voltage of 10kV. Transmission Electron Microscopy (TEM) studies were carried out on a JEM2100 instrument. All electrochemical experiments like, cyclic voltammetry, galvanostatic charge-discharge, electrochemical impedance spectroscopy (EIS) and cycling studies were performed in 6M KOH solution aqueous electrolyte and commercial organic electrolyte using a biologic SP300 electrochemical workstation. All the electrochemical measurements were calculated in the room temperature.
Carbonization of Bamboo Bagasse
The bamboo bagasse was first washed with water to remove all the impurities and contaminants in it. Then it is washed with ethanol and kept for drying under the sunlight for 2 days. They were cut into small pieces, and those pieces was treated into the muffle furnace at 300°C for 2 hours and cooled for 24 hours and thus the char (carbon) was obtained. The carbon powder obtained was finely crushed by the ball-mixing process.
Activation of Carbon Derived from Bamboo Bagasse
Zinc chloride is used the activating agent as it prevents the accumulation of tar on the carbon surface and provides further decompositions and thus, develops the micro-porosity. The biomass-derived carbonized power was mixed with ZnCl2 at the ratio of 1:0.5 under stirring to form a completely homogeneous solution with ethanol as the solvent. It was kept into the Teflon lined autoclave for about 24 hours for 180°C. Then, it was treated into the tubular furnace for 800°C for 2 hours under the flow of carbon-di-oxide inert gas. The obtained activated carbon was washed with 10% hydrochloric acid (HCl) and deionized many times to eliminate metal ions and other fragments of elements such as oxygen, water,-CH2 and the solution becomes neutral. Then, it was vacuum dried at 120°C and hence ZnCl2 catalyzed activated carbon was obtained.
Preparation of Electrode Material
The performance of capacitance for the supercapacitor system was investigated by fabricating the two electrode coin cell system. The electrode material for the coin cell is prepared by homogeneously mixing 85% of active prepared materials, 10% of conducting carbon and the 5% of Teflon solution and the solvent used is the iso-propyl alcohol. It is mix until it becomes a pasty or slurry-like substance. The paste was spread onto a butter paper and dried at 120°C for 2 hours, then weighed and pressed under the pressure of 50-100 MPa. The active mass loading of the electrode is approximately 3-5 mg. Two electrodes were fitted with the separator and aqueous and non-aqueous electrolyte was assembled symmetrically into sandwich type cell.
Result and Discussion
Physical Characterization
The prepared carbon material was subjected to physical characterization technique such as X-Ray diffraction. The surface morphology was characterized using the scanning electron microscopy and Transmission Electron Microscope.
X-Ray Diffraction Spectroscopy
This technique is used to determine the crystal structure of the material. The XRD spectrum of the zinc-catalysed prepared carbon is shown in figure 1. The carbon has the 002 and 101 plane at 23.8° and 45°, which was not observed when treated with iron, reported in the previous paper.1 The intensity of the peak is high which suggests the material is highly crystalline in nature. The sharp peak in the (002) plane provides the information that there is an in-plane conductivity and that is very much needed for the better performance of the electrochemical properties of the material. The d-spacing of both the planes were calculated and found to be 3.81 and 2.01 respectively. Thus the result shows that the carbon obtained from the biomass (bamboo bagasse) when treated with zinc, it becomes more crystalline but not when treated with iron salt.
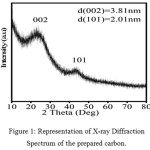 |
Figure 1: Representation of X-ray Diffraction Spectrum of the prepared carbon. |
Scanning Electron Microscopy (SEM)
The prepared activated carbon material was evaluated for the surface properties by employing SEM with EDAX and TEM. It is depicted in Figure 2(a-f). The picture represents from lower magnification to higher magnification. From the images, it is clearly identified that more 3D interconnected micro-pores are formed after the activation of zinc chloride. The iron catalysed carbon in the previous study showed only paper like surface morphology1. Surprisingly, when the same carbon was catalysed with zinc chloride, at 50µm, the flake-like morphology is obtained. The micro-pores are formed between the flakes. It is interesting, to see the uniform and homogenous porosity formed in the surface of the prepared activated carbon during the carbonization and activation process which helps in the enhancement of electrochemical properties of the material. The size of the pores is determined and was found to be (3.7 µm *4.1 µm). The TEM image at lower magnification shows a crumbled paper like morphology. The SAED pattern clearly depicts the (002) and (101) plane which is shown in the XRD spectrum. The EDAX pattern shows the presence of little amount of zinc particle, which improves the electrochemical activity of the prepared carbon. Due to the formation of pores, the adsorption sites for the solute used for the electrochemical experiments is increased which makes the property improved.14-15 From this surface property result, this type of structured material can be opted as an alternative energy conversion electrode which gives better and improved performance.
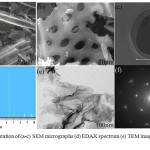 |
Figure 2: Representation of (a-c) SEM micrographs (d) EDAX spectrum (e) TEM images (f) SAED pattern. |
Electrochemical Measurements
In order to evaluate the electrochemical properties of the prepared zinc- catalyzed activated carbon, all the electrochemical measurements were calculated using different electrochemical techniques like cyclic voltammetry, galvanostatic charge-discharge studies, electrochemical impedance spectroscopy and cycling studies. All the above study was carried out under ambient temperature and pressure conditions. Two-electrode symmetric system (coin cell) was employed to study the electrochemical measurements. The entire study was carried out in both the aqueous (6M KOH) and Non-Aqueous (Et4NBF4) under room temperature.
 |
Figure 3: (a-b) Cyclic voltammetry curve of aqueous and non-aqueous (b) galvanostatic charge discharge profile of aqueous and organic electrolyte. |
In cyclic voltammetry study, 0V to 1V and 0V to 2.7V is applied as the potential for KOH and Et4NBF4 electrolyte. The study is carried out for different scan rates (20 mVs-1, 40 mVs-1, 50 mVs-1 and 100 mVs-1) and is depicted in figure 3(a,b). The mass of the electrode is around 5 mg. The curves formed in the study are of rectangular shape and found to be in good symmetry even at the higher scan rate of 100 mVs-1. This indicates the higher specific capacitance and good rate properties. The maximum specific capacitance was found to be 180 Fg-1 and 153 Fg-1 for aqueous and non-aqueous electrolyte, respectively. Due to the high viscosity, low electrical conductivity and high equivalent series resistance (ESR) of the non-aqueous electrolyte, it obtains a lower capacitance values than the aqueous electrolyte
To survey the rate capability of the prepared carbon it is subjected to charge-discharge at different current density such as 1 Ag-1, 2 Ag-1, 5 Ag-1 and 10 Ag-1. It is shown in figure 3(c,d). From the charge/discharge graph, the maximum specific capacitance was found to be, 180 Fg-1 and 153 Fg-1 for aqueous electrolyte and non-electrolyte at 1 Ag-1, respectively. The identical shape of the charge/discharge profile at various current densities retains a proficient charge and ion transfer process.
The Specific Capacitance was calculated using the following formula,
![]()
The energy density and power density is calculated using the formula;
Energy Density = 0.5* Specific Capacitance * Potential Window2 Eq.2
![]()
The aqueous specific capacitance were obtained to be 180 Fg-1, 178 Fg-1, 176.3 Fg-1, 174.2 Fg-1, energy density were obtained as 12.8 Whkg-1, 10.4 Whkg-1, 8.7 Whkg-1, 6.7 Whkg-1 and power density was found to be 1.7 kWkg-1, 2.2 kWkg-1, 2.5 kWkg-1, 2.7 kWkg-1 at different current densities 1Ag-1, 2Ag-1, 5A-1 and 10Ag-1 respectively. The non-aqueous specific capacitance was obtained to be 153 Fg-1, 106 Fg-1, 100.3 Fg-1, 95 Fg-1, energy density was obtained as 35 Whkg-1, 32 Whkg-1, 20 Whkg-1, 11 Whkg-1 and power density was found as 1 kWkg-1, 1.4 kWkg-1, 1.9 kWkg-1, 2.3 kWkg-1 at different current densities 1 Ag-1, 2 Ag-1, 5 A-1 and 10 Ag-1 respectively.
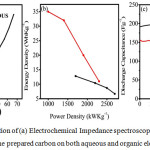 |
Figure 4: Representation of (a) Electrochemical Impedance spectroscopy (b) Ragon’s Plot and (c) cycling study of the prepared carbon on both aqueous and organic electrolyte. |
The figure 4(a) reveals the electrochemical impedance spectroscopy of Zinc Chloride- activated carbon in both aqueous and non-aqueous electrolyte. In the electrochemical impedance spectroscopy (EIS) spectrum, the semicircle which is elongated, tells about the structure of distribution of pores. EIS was subjected within the range of 100kHz to 10 MHz with 10mV amplitude. The EIS plot shows linearity, which depicts that control of the diffuse process of the electrode material, can be controlled and studied. Electric double layer (EDL) formation is represented by the slope of these lines. The Rs (internal resistance) in aqueous and non-aqueous was found to be approximately 5.5 Ω, 1.57 Ω, respectively. The Rct (charge transfer resistance) in aqueous and non-aqueous medium was obtained to be approximately 25.4 Ω and 6.4 Ω, respectively. The Ragone’s plot depicts the correlation between the obtained energy density and the power density which is depicted in the figure 4(b). It shows linear correlation and hence it suggests that the prepared carbon material is of good electrochemical performance. The cycling studies were carried out for the prepared carbon in both aqueous and non-aqueous electrolyte for 10,000 cycles which is depicted in figure 4(c). The capacitance retention was found to be 94% for the aqueous electrolyte and 85% for the non-aqueous electrolyte. The result reveals the superior life stability of the prepared carbon material.
Conclusion
The zinc chloride- catalysed activated carbon from the biomass was succesfully synthesized and applied for the supercapacitor application. The pores were not formed during the iron catalsation in the previous paper, but here the pores are generated when catalysed by the zinc chloride. The prepared carbon material showed improved specific capacitance, energy density and power density than the iron catalysed carbon material which was reported in the previous work. It is concluded that there is 20 % increase in the electrochemical performace than with iron-catalysd carbon. The maximum specific capacitance of 180 Fg-1 and 153 Fg-1 was obtained for aqueous and non-aqueous electrolyte. The maximum energy density of the aqueous medium and is obtained to be around 12.8 Whkg-1 at 1 Ag-1 current density and the maximum power density of the aqueous electrolyte was found to be 2.7 kWkg-1 at 10 Ag-1 current density. The maximum energy density of the non-aqueous medium and is obtained to be around 35 Whkg-1 at 1 Ag-1 current density and the maximum power density of the non-aqueous electrolyte was found to be 2.3 kWkg-1 at 10 Ag-1 current density. This method of catalyzing the carbon with zinc chloride, makes the carbon more electrochemically active and enhances their performances than the other activating agents. This method of combined hydrothermal and chemical activation process can be an effective in the field of carbon science and technology.
Acknowledgements
The first author (Sivagaami Sundari Gunasekaran) is gratefully acknowledges the financial support from VISTAS, Grant Vels Research Fellowship (VRF).
References
- Gunasekaran, S. S., Elumalali, S. K., Kumaresan, T. K., Meganathan, R., Ashok, A., Pawar, V., & Bose, R. S., Materials Letters, 2018, 218, 165-168.
CrossRef - Kötz, R., & Carlen, M, Principles and applications of electrochemical capacitors. Electrochimica acta, 2000, 45(15-16), 2483-2498.
CrossRef - B. E, Scientific Fundamentals and Technological Applications, 1999.
- Burke, A., Journal of power sources, 2000, 91(1), 37-50.
CrossRef - Arbizzani, C., Gallazzi, M. C., Mastragostino, M., Rossi, M., & Soavi, F, Electrochemistry Communications, 2001, 3(1), 16-19.
CrossRef - Choi, D., Blomgren, G. E., & Kumta, P. N, Advanced Materials, 2001, 18(9), 1178-1182.
CrossRef - Frackowiak, E., & Beguin, F, Carbon, 2001, 39(6), 937-950.
CrossRef - Ruiz, V., Blanco, C., Granda, M., Menéndez, R., & Santamaría, R, Journal of applied electrochemistry, 2007, 37(6), 717-721.
CrossRef - Ruiz, V., Blanco, C., Granda, M., Menéndez, R., & Santamaría, R, Journal of applied electrochemistry, 2007, 37(6), 717-721.
CrossRef - Yoon, S., Lee, J., Hyeon, T., & Oh, S. M, Journal of the Electrochemical Society, 2000, 147(7), 2507-2512.
CrossRef - Weng, T. C., & Teng, H, Journal of the Electrochemical Society, 2001, 148(4), A368-A373.
CrossRef - Gamby, J., Taberna, P. L., Simon, P., Fauvarque, J. F., & Chesneau, M, Journal of power sources, 2001, 101(1), 109-116.
CrossRef - Molina-Sabio, M., & Rodrıguez-Reinoso, F, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 241(1-3), 15-25.
CrossRef - El-Naggar, A. H., Alzhrani, A. K., Ahmad, M., Usman, A. R., Mohan, D., Ok, Y. S., & Al-Wabel, M. I., BioResources, 2015, 11(1), 1092-1107.
CrossRef - Vold, R. D., & Vold, M. J., Colloid and interface chemistry, 1983, 322-323.

This work is licensed under a Creative Commons Attribution 4.0 International License.









