Effect of Nickel and Molybdenum on the Condition of Oxide-Zirconium Coatings on Steel Base
Murat Zhurinov1, Vadim Statsyuk1, Lidiya Fogel1, Amangul Bold1 , Ularbek Sultanbek1, Alexey Abrashov2 and Anna Kalinkina2
, Ularbek Sultanbek1, Alexey Abrashov2 and Anna Kalinkina2
1D.V. Sokol’skiy Institute of Fuels, Catalysis and Electrochemistry, Almaty, Kazakhstan.
2D.I. Mendeleev University of Chemical Technology of Russia, Moscow, Russia.
Corresponding Author E-mail: b.amangul@inbox.ru
DOI : http://dx.doi.org/10.13005/ojc/350131
Article Received on : 21-11-2018
Article Accepted on : 06-02-2019
Article Published : 25 Feb 2019
In this paper, we developed the conditions for the deposition of zirconium oxide coatings from solutions containing hexafluorozirconic acid, nickel and molybdenum salts on a steel base. Optimal concentrations of nickel and molybdenum were determined to obtain oxide-zirconium coatings used as adsorption layers for paint coatings. The effect of temperature, time and subsequent heat treatment on the protective anticorrosive and adhesive characteristics of oxide-zirconium coatings has been established. It is shown that the formed oxide-zirconium coating are Nano scale size. It is established that the corrosion resistance of the investigated coatings in the temperature range 30-40°C is in agreement with their adhesion strength. On the basis of electrochemical studies, it was shown that the addition of hexafluorozirconic acid to nickel and molybdenum salts leads to inhibition of iron ionization and an increase in the corrosion resistance of the formed coating.
KEYWORDS:Cyclic Current-Voltage Curves; Hexafluorozirconic Acid; Molybdenum; Nickel; Oxide-Zirconium Coatings; Protective Ability
Download this article as:| Copy the following to cite this article: Zhurinov M, Statsyuk V, Fogel L, Bold A, Sultanbek U, Abrashov A, Kalinkina A. Effect of Nickel and Molybdenum on the Condition of Oxide-Zirconium Coatings on Steel Base. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Zhurinov M, Statsyuk V, Fogel L, Bold A, Sultanbek U, Abrashov A, Kalinkina A. Effect of Nickel and Molybdenum on the Condition of Oxide-Zirconium Coatings on Steel Base. Orient J Chem 2019;35(1). Available from: https://bit.ly/2SY7bgv |
Introduction
Modern technologies of applying paint coatings on metal surfaces suggest the preliminary application of conversion coatings. Converse coatings are applied to metals to increase paint adhesion and increase corrosion resistance. These coatings are usually formed as a result of an electrochemical process without external current. For many years, phosphate conversion coatings have been used to protect metal structures, since they provide excellent corrosion resistance for ferrous and non-ferrous alloys.1 However, phosphating process and implementation of advanced technologies for producing phosphating coatings is not perfect for effects on the environment. The main disadvantages of phosphating processes widely used in industry are: the content of toxic nickel ions, nitrite ion, etc.; high energy intensity due to high working temperatures of processes 70-90°С; the release of hydrogen, which prevents the depositions formation, high slime formation, the need for sophisticated equipment and strict control of deposition parameters.2-5 Today, an important task is to find a suitable replacement for phosphate processing, capable of providing the same or even better adhesion than traditional methods of phosphating. In recent years in world practice, nanostructured ceramic coatings have been increasingly used as an alternative to conversion phosphate layers,6-15 of which the most promising are Zr-conversion coatings. Solutions for these coatings do not require heating, strict control parameters, are easy to apply, to form a much smaller sludge and more environmentally friendly. The process of oxide-zirconium coatings deposition consists of the hexafluorozirconic acid effects on the metal surface, which leads to the formation of a conversion coating mainly consisting of ZrO2 and having a thickness less than 100 nm. From the literature11 it is known that solutions for the deposition of oxide-zirconium coatings should contain heavy metal ions, which, precipitating contact on the surface of the steel base, initiate the deposition of conversion Zr-containing layers.In view of this, solutions containing, in addition to hexafluorozirconic acid (H2ZrF6), Ni (II) and Mo (VI) ions, which were introduced into the solution in the form of their respective salts Ni(NO3)2·6H2O and (NH4)6Mo7O24 as the object of study were selected.
Materials and Methods
Plates of cold-rolled steel of grade (Art. 08 PS) were used as samples. For an accelerated assessment of the coatingsprotective ability a rapid method using the Asimov’s reagent – a solution, containing CuSO4·5H2O; NaCl; HCl,16 was used. According to this method, the protective ability of the coating (ASA) is estimated in seconds as the time of change in the color of the control area under a drop of solution from gray to red-brown. Corrosion tests of adhesive coatings with polyester powder paint were carried out in an Alcott S450 iP salt fog chamber in accordance with the international standard ASTM B117 adopted in the automotive industry.17 The coating thickness was determined by the ellipsometric method using a Sentech SEN reseach 4.0 SER 800 spectroscopic ellipsometer with a high-speed monochromatеr. Measurements were performed in the spectral range of light wavelengths 240–1000 nm. The adhesion strength of the coatings was determined by the method of normal separation (the method of fungi) using a digital adhesiometer Posi Test AT. The method is based on measuring the minimum breaking stress required to separate or tear the coating in the direction perpendicular to the substrate surface. Electrochemical studies included cyclic voltammetry. Cyclic current-voltage curves were taken on a potentiostat-galvanostat Gamry Reference 3000 (USA), in a sealed three-electrode cell at 25°С. The working electrode was an iron electrode St-3 with a visible surface of 0.03 cm2. The counter electrode was a platinum electrode with a surface of 2 cm2; Ag/AgCI electrode, whose potential is 196 mV relative to the hydrogen electrode, was used as a reference electrode. Before fixing the cyclic current-voltage curves, the working electrode surface was updated with MIRKA 2000 emery paper, washed with distilled water, then polished on a paper filter (blue tape) and finally washed with distilled water. Theused electrolyte was a solution of 0.3M Na2SO4. The morphology and surface relief of the obtained coatings were studied using a JSMU-3 scanning electron microscope (JEOL, Japan).
Results and Discussion
The study of influenc eof nickel salt concentration added to the solution of hexafluorozirconic acid on the coating corrosion resistance by Asimov’s method was completed. Figure 1 shows the dependence of the oxide-zirconium coatings corrosion resistance on the concentration of Ni(NO3)2*6H2O in solution for coating deposition, containing 1.5 g/l H2ZrF6 and different concentrations of Ni(NO3) 2*6H2O, pH – 4.0-5.5.
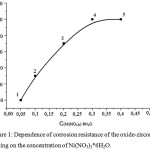 |
Figure 1: Dependence of corrosion resistance of the oxide-zirconium coating on the concentration of Ni(NO3)2*6H2O. |
According to Figure 1 with increasing concentrations of Ni(NO3)2*6H2O from 0.05-0.5 g/l the degree of zirconium oxide coating corrosion resistance increases, reaching a value of 180 c at a concentration of Ni(II) – 0.35-0.40 g/l. Thus, the optimal concentration of nickel salt is 0.35 – 0.40 g/l.
A study of the effect of (NH4)6Mo7O24 concentration added to the deposition solution on the corrosion resistance of the oxide-zirconium coatings is shown in Figure 2. The coating deposition solution contains H2ZrF6 – 1.5 g/l; Ni (NO3)2*6H2O – 0.4 g/l; pH – 4.0-5.5.
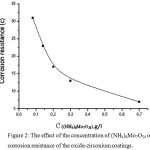 |
Figure 2: The effect of the concentration of (NH4)6Mo7O24 on corrosion resistance of the oxide-zirconium coatings. |
The experimental data revealed that increasing the concentration of (NH4)6Mo7O24 reduces the protective capability of the coatings, but the presence of this compound in the used solution contributes to the formation of more uniform coatings. Figure 3 shows micro graphs of steel samples without coating (a) and in the presence of an oxide-zirconium coating from solutions containing the salt of nickel (b) and molybdenum (c).
 |
Figure 3: Micrographs of an uncoated iron sample (a) with a zirconium oxide coating in the presence of Ni(II) (b) and Mo(VI) ions (c). |
According to Figure 3 the presence of a zirconium oxide coating leads to a changing the staining of steel sample surface. When Ni (II) ions are added to the used solution Figure 3(b), the surface gets a yellowish-blue shade. When Mo (VI) ions are added to the used solution Figure 3(c), the coating becomes yellow coloring and surface structure becomes more uniform.
Ellipsometric measurements made it possible to determine the thickness of the oxide-zirconium coatings having a nano-dimensional value. So, the thickness of the oxide-zirconium coatings obtained from a solution containing of Ni(NO3)2*6H2O is ~ 9.2 nm, and in addition to (NH4)6Mo7O24 in the solution used, the thickness is ~ 83 nm.
The thickness of the coating is largely determined by the deposition temperature. Figure 4 shows the dependence of the thickness of the formed oxide-zirconium coatings on their temperature deposition in solution: 1,5 g/l(H2ZrF6) + 0,4 g/lNi(NO3)2*6H2O + 0,08 g/l(NH4)6Mo7O24, pH – 4.0-5.5, precipitation time (τ) – 3 min.
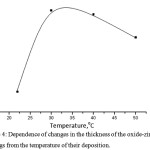 |
Figure 4: Dependence of changes in the thickness of the oxide-zirconium coatings from the temperature of their deposition. |
According to Figure 4, with an increase in the deposition temperature, the thickness of the oxide-zirconium coatings grows and at 30°C is 94.7 nm. With a further increase in temperature, the thickness of the coatings becomes less at 40°C – 94.3 nm, at 50°C – 91.47 nm. In view of these results the temperature of 30°C was taken as the optimum process temperature.
The optimal values of temperature and time of deposition of oxide-zirconium coatings were determined for the value of their protective ability (Figure 5).
According to Figure 5, the optimum deposition time of the oxide-phosphate coating at a temperature of 30°C is 10 min., at 40°C – 5 min., at 50°C – 5 min. Thus, for optimal conditions for the formation of oxide-zirconium coatings, a time of 10 minutes is accepted at a temperature of 30°C.
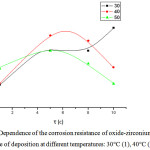 |
Figure 5: Dependence of the corrosion resistance of oxide-zirconium coatings on the time of deposition at different temperatures: 30°C (1), 40°C (2), 50°C (3). |
The subsequent heat treatment of the oxide-zirconium coatings significantly affects of their corrosion resistance. Figure 6 shows the influence of temperature subsequent drying zirconium oxide coatings on their corrosion resistance in solution: 1,5 g/l (H2ZrF6) + 0,4 g/lNi(NO3)2 *6H2O + 0,08 g/l (NH4)6Mo7O24, pH – 4.0-5.5, precipitation time (τ) – 3 min.
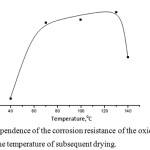 |
Figure 6: Dependence of the corrosion resistance of the oxide-zirconium coating on the temperature of subsequent drying. |
According to Figure 6, the maximum protective ability of zirconium oxide coatings is observed in the temperature range from 70 to 130°C. A higher drying temperature range leads to a decrease in protective ability.
An important factor possibility of the obtained zirconium oxide coatings use of is their adhesive strength, which is determined by the adhesive strength of coatings to the steel substrate. For the quantitative determination of the adhesion strength used the method of normal separation (method of fungi). Table 1 shows the experimental data to determine the adhesive strength of oxide-zirconium coatings at different temperatures of their deposition.
Table 1: The effect of temperature and time of deposition on the speed on the strength of oxide-no-zirconium coatings.
|
Temperature(t°С) |
22 |
30 |
40 |
50 |
|
Optimal deposition time (τ, min.) |
3 |
10 |
3 |
5 |
|
Speed of separation (MPa/s) |
3,03 |
3,30 |
4,63 |
4,04 |
From the data in Table 1 shows that the rate of separation of zirconium oxide coating from the iron surface of the samples in the temperature range from 22 to 50°C reaches a maximum value of 4.63 MPa /s at 40°C.
Corrosion tests on steel samples with an oxide-zirconium coating and paint were carried out in a salt fog chamber. The test samples were painted with a cruciform incision to the metal substrate of 0.5 mm in width.Samples of oxide-zirconium coatings after testing in the salt fog chamber at different temperatures are shown in Figure 7.
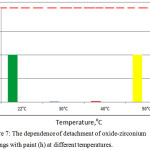 |
Figure 7: The dependence of detachment of oxide-zirconium coatings with paint (h) at different temperatures. |
According to Figure 7, the depth of corrosion penetration in cuts of steel samples with oxide-zirconium coatings obtained at 22°C and 50°C is 1 mm; at 30°C and 40°C, this value is almost 0. Thus, tests in the salt fog chamber indicate that the optimum temperature for deposition of oxide-zirconium coatings, at which the highest corrosion resistance is observed, is 30-40°C, which is in agreement with the results of determining the adhesive strength of the coatings obtained. Thus, testing in the salt fog chamber indicate that the optimum deposition temperature of the zirconium oxide coatings, at which a maximum corrosion resistance is observed, is 30-40°C, which is in agreement with the results of the coatings adhesive strength determination.
To obtain independent information about the processes occurring on iron samples during the deposition of oxide-zirconium coatings, the cyclic volt-ampere curves were obtained on an iron electrode in a solution of 0.3 M Na2SO4 in the presence of hexafluorozirconic acid and Ni(II) and Mo(VI) ions (Figure 8).
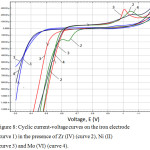 |
Figure 8: Cyclic current-voltage curves on the iron electrode (curve 1) in the presence of Zr (IV) (curve 2), Ni (II) (curve 3) and Mo (VI) (curve 4). |
Cyclic current-voltage curves were obtained in the potential range from -0.3 to -1.2 V in 0.3 M Na2SO4 (curve 1). According to Figure 1, the presence of 2.88•10–4 M hexafluorozirconic acid(curve 2) in the solution leads to the appearance of a cathode current maximum at E = –1.04 V, caused by the reduction of Zr(VI) ions on the iron electrode surface, as well as anode maximum current at E = -0.76 V, due to oxidation of reduction products. Adding 1×10–4 M nickel salt to the solution (curve 3) leads to a displacement of the potential of the cathode maximum current E = –1.0 V, due to the simultaneous reduction of nickel and zirconium ions, and to the absence of an anode maximum current. At the same time, the ionization potential of the iron electrode is shifted to the anode region. Further addition of the 1.7•10–6 M molybdenum salt to the solution (curve 4) helps to reduce the current of the cathode maximum due to the simultaneous reduction of Zr (IV), Ni (II) and Mo (VI) ions, and to the displacement of its potential (E = – 1.05 V).In the anodic region, a significant displacement of the ionization potential is observed towards less negative potentials, which testify to the inhibition of the corrosion process.
Thus, on the basis of cyclic current-voltage curves, it was shown that the addition of hexafluorozirconic acid to nickel and molybdenum salts in solution leads to inhibition of the anodic process of ionization of the iron electrode and an increase in the corrosion resistance of the coating being formed. Thus, based on the cyclic voltammetric curves shows that the addition to hexafluorozirconic acid solution of nickel and molybdenum salts leads to inhibition of the iron ionization and increase the corrosion resistance of the formed coating.
Conclusion
The optimal conditions for the deposition of oxide-zirconium coatings used as adsorption layers for paint coatings have been developed from solutions containing hexafluorozirconic acid, nickel and molybdenum salts on a steel base.It is shown that the formed oxide-zirconium coatings have a nanoscale value. In the presence of nickel salts thickness is 92 nm and with the addition of molybdenum salt in solution obtained uniform coating with a thickness of 83 nm. It is established that the corrosion resistance of the investigated coatings in the temperature range 30°-40°C is in agreement with their adhesion strength under similar temperature conditions.Based on electrochemical studies, it was shown that the addition of nickel and molybdenum salts to the solution to precipitate oxide-zirconium coatings leads to inhibition of the anodic process of iron ionization.
Acknowledgements
The work was conducted under research grand project ofАР05132222″Development of resource-saving technology for applying ceramic adhesive nanocoats with improved characteristics”. The authors would like to place on record their sincere gratitude to the Ministry of Science and Education of the Republic of Kazakhstan for financial support.
References
- Abrashov, A.A.; Chamashkina, N.N.; Yur’eva, G.A.; Grigoryan, N.S.; Vagramyan, T.A. Electroplating and surface treatment. 2012, 4(20), 7-12.
- Abrashov, A.A.; Grigoryan, N.S.; Vagramyan, T.A.; Akimova, E.F.Electroplating and surface treatment. 2010, 3(18), 48-52.
- Kulyushina, N.V.;Grigoryan, N.S.; Mazurova, D.V.; Kalinkina, A.A.; Men’shikov, V.V., Va-gramyan, T.A. Protection of Metals and Phisical Chemistry of Surfaces. 2011, 7(47), 884-888.
CrossRef - Abrashov, A.A.; Grigoryan, N.S.; Vagramyan, T.A.; Papirov R.V.; Styazhkina, M.I. Electroplating and surface treatment. 2013, 4(21), 40-45.
- Statsyuk, V. N.; Sultanbek, U.; Fogel, L.A. News of NAN RK (ser. Chem.). 2016, 5, 197-199.
- Pat. RU 2622076 C1, publ. 09.07.2017. Abrashov, A.A.; Vagramyan, T.A.; Grigoryan, N.S.
- Abrashov, A. A.; Grigoryan, N.S.; Nazarova, G.A.; Solod, L.O.; Vagramyan, T.A. Science and world. International scientific journal. 2015, 11(27), 65-67.
- Abrashov, A. A.; Grigoryan, N.S.; Vagramyan, T.A.; Zhilenko, D.Y. Non-ferrous Metals. 2016, 11, 33-37.
CrossRef - Metroke, T.; Kachurina, O.; Knobbe, E. JCT Coat. Technol. 2002, 74, 927.
CrossRef - Dunham, B.; Chalk, D.; Sharonville, L.; Ohio, M. Cleaning, pretreatment & surface preparation. 2012, 5, 112-118.
- SaikatAdhikaria, K.A.; Unocica, Y; Zhaia, G.S.; Frankela, J.; Zimmermanb, W. ElectrochimicaActa. 2011, 56, 1912-1924.
- Ghanbari,A.; Attar, M.M. Surface & Coatings Technology. 2014, 246, 26–33.
CrossRef - Hossein Eivaz Mohammadloo, Ali Asghar Sarabi, Ali Asghar Sabbagh Alvani, Hassan Sameiea, Reza Salim, Surf. Coat.Technol. 2012, 206, 4132–4139.
CrossRef - Yi, A.H.; Li,W.; Du, J.; Mu, S.L. App. Surf. Science. 2012, 258, 5960-5964.
CrossRef - Tepe, B.; Gunay, B. Progress in Organic Coatings. 2008, 62, 134-144.
CrossRef - GOST 9.302-88. ESZKS. Metallic and Nonmetallic Coatings.Methods of Accelerated Corrosion Tests.
- GOST 9.401-91. ESZKS. Lacquer Coatings. General Requirements and Methods of Accelerated Tests for Stability to Effect of Climatic Factors, Method B “Determination of Stability of Coatings to the Effect of Salt Mist (Development of Corrosion from a Cut)”.

This work is licensed under a Creative Commons Attribution 4.0 International License.









