Catalytic Oxidation of C3-C4 Mixture Into Industrial Important Chemical Products
Tolkyn Baizhumanova*1,2 , Svetlana Tungatarova1,2, Gulnar Kaumenova1,2, Manapkhan Zhumabek1 and Kaysar Kassymkan1
, Svetlana Tungatarova1,2, Gulnar Kaumenova1,2, Manapkhan Zhumabek1 and Kaysar Kassymkan1
1D.V. Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142 Kunaev str., Almaty, 050010, Republic of Kazakhstan.
2Al-Farabi Kazakh National University, Almaty, Kazakhstan.
Corresponding Author E-mail: baizhuma@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/350151
Article Received on : 28-09-2018
Article Accepted on : 22-01-2019
Article Published : 18 Feb 2019
The results of a study of the activity of polyoxide catalysts based on molybdenum, chromium and gallium supported on natural clays for the catalytic oxidation of light alkanes to industrially important chemical products are presented. Studies of the oxidative conversion of a propane-butane mixture on molybdenum, chromium and gallium polyoxometallates supported on natural clays allowed to determine that the predominant composition of the products is determined by the temperature of the process. It was found that the production of a number of products with high yields: acetaldehyde - at 673-723 K, acetone - at 823 K, methanol - at 673-723 K, MEK - at 773-823 K, ethanol - at 823 K, ethylene - at 673 -723 K, H2 - at 823 K is possible at oxidative conversion of propane-butane mixture at GHSV = 450 h-1 on the developed three-component supported Mo-Cr-Ga catalysts.
KEYWORDS:Catalytic Oxidation; Catalysts; C3-C4 Mixture
Download this article as:| Copy the following to cite this article: Baizhumanova T, Tungatarova S, Kaumenova G, Zhumabek M, Kassymkan K. Catalytic Oxidation of C3-C4 Mixture Into Industrial Important Chemical Products. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Baizhumanova T, Tungatarova S, Kaumenova G, Zhumabek M, Kassymkan K. Catalytic Oxidation of C3-C4 Mixture Into Industrial Important Chemical Products. Orient J Chem 2019;35(1). Available from: https://bit.ly/2Ehfuek |
Introduction
Natural and oil gases are the most important alternative sources of raw materials, capable in the long term to compete with oil. The problem of rational use of C1-C4 alkanes, included in their composition, is particularly relevant in countries rich in this type of raw materials. Despite the huge reserves of hydrocarbon raw materials in the Republic of Kazakhstan, it is mainly spent in the form of domestic, industrial and motor fuel, and the remaining raw materials are burned as part of exhaust gases, or again pumped into oil reservoirs. In this regard, the processing of alkanes (the main components of natural gas and oil) for the purpose of obtaining industrially important chemical products is one of the most urgent environmental problems in Kazakhstan.
Formation of synthesis gas, unsaturated hydrocarbons, aldehydes, acids and alcohols should be expected at incomplete oxidation of methane, ethane, propane and butane. Only the optimal selection of catalysts can purposefully to carry out the process with preferential formation one of the listed products. However, the development of new effective catalysts for selective oxidation of light alkanes is still at the stage of research and development.1
In the process of oxidative conversion of propane-butane mixture on various types of catalysts is possible to obtain a range of products such as oxygenates,2-5 olefins,6-9 hydrogen,10,11 synthesis gas12-14 and CO2 + H2O.15
Experimental
Catalyst Preparation
Preliminary preparation was carried out for the preparation of catalysts on natural carriers. The natural carriers were dried at 473 K for 2 h, calcined at 773 K for 2 h and then treated in a solution of 10% HCl and calcined again at 773 K for 2 h. The catalysts were prepared by the capillary impregnation method of mixed aqueous solutions of nitrate salts of metals, supported on preformed natural clays.
Characterization Techniques
The analysis of the initial mixture and reaction products was carried out using a chromatograph “Chromos GC-1000” with the “Chromos” software and on a chromatograph “Agilent Technologies 6890N” (USA) with computer software. Chromatograph “Chromos GC-1000” is equipped with packed and capillary columns. The packed column is used for the analysis of Н2, О2, N2, СН4, С2Н6, С2Н4, С3-С4 hydrocarbons, СО and СО2. A capillary column is used to analyze of liquid organic substances, such as alcohols, acids, aldehydes, ketones and aromatic hydrocarbons. Temperature of the detector by thermal conductivity – 200°С, evaporator temperature – 280°С, column temperature – 40°С. Carrier gas velocity Ar = 10 ml/min. The chromatographic peaks were calculated from the calibration curves plotted for the respective products using the “Chromos” software for pure substances. Based on the measured areas of the peaks corresponding to the amount of the introduced substance, a calibration curve V = f (S) was constructed, where V – amount of substance in ml, S – peak area in cm2. Concentrations of the obtained products were determined on the basis of the obtained calibration curves. The balance of regulatory substances and products was ± 3.0%.
Physico-Chemical Research
The phase composition of catalysts was determined on X-ray diffractometer DRON-4-7 with Co-anode (25 kV, 25 mA, 2θ = 15-80°).
Determination of the surface was conducted by low-temperature adsorption of nitrogen by the BET method on the “Accu Sorb” installation from Micromeritics produced in the USA.
Morphology, particles size, chemical composition of initial and worked out catalysts were performed on transmission electron microscope TEM-125K with enlargement up to 66000 times by replica method with extraction and micro diffraction. Carbonic replicas were sputtered in vacuum universal station, and carrier of catalysts was dissolved in HF. Identification of micro diffraction patterns were carried out by means of ASTM cart index (1986).
Results and Discussion
The results of a study of activity of the Mo-Cr-Ga catalysts supported on natural clays of different content in the process of partial oxidation of propane-butane mixture at С3-С4 : О2 : N2 : Ar = (33.33 : 7.0 : 26.34 : 33.33, %), GHSV = 450 h-1 and a temperature range of 673-823 K are presented. As can be seen from Figure 1, the formation of C2H4, H2, and CO2 was observed at oxidative conversion of propane-butane mixture on the developed 1% MoCrGa catalyst. A 39.2% yield of ethylene passed through a maximum at 773 K. The formation of 1.0 – 2.6% H2 was also observed. 39.5% of CO2 is formed by raising the reaction temperature to 773 K and up to 823 K in reaction mixture. With increasing temperature, the process proceeds towards the formation of a deep oxidation product (CO2) along with oxidative dehydrogenation (C2H4, H2).
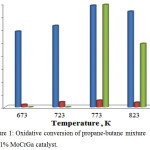 |
Figure 1: Oxidative conversion of propane-butane mixture on 1% MoCrGa catalyst. |
Figure 2 shows the activity of 5% MoCrGa/TWC catalyst at oxidative conversion of propane-butane mixture. The greatest yields of acetaldehyde and methanol were obtained at relatively low temperatures of 673-723K. The yield of acetaldehyde decreased from 33.3% to 11.5% with an increase in temperature from 673 K to 823 K, and methanol – from 13.8% to 10.7% at 773 K. Methanol was not detected in product at higher temperatures. A different picture was observed for acetone and methyl ethyl ketone. The maximum yields for these products were observed at 823 K. The yield of acetone at this temperature was 50.9%, and methyl ethyl ketone – 37.6%. It was determined that content of ethylene at all temperatures decreased compared with 1% catalyst. However, the yield of hydrogen increased from 5.7 to 18.8%. The formation of products of deep oxidation was not observed.
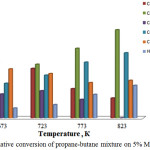 |
Figure 2: Oxidative conversion of propane-butane mixture on 5% MoCrGa catalyst. |
The same trend of reduction of gaseous substances in reaction products was observed at a further increase in the content of MoCrGa on the carrier to 10%. The yield of ethylene did not exceed 8.6%. A new product – ethanol appeared in the liquid phase, the yield of which was 66.0% at 823 K.
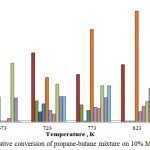 |
Figure 3: Oxidative conversion of propane-butane mixture on 10% MoCrGa catalyst. |
It can be seen from the data in Figure 3 that the decrease in the formation of acetaldehyde, acetone, methanol, MEK, ethylene and hydrogen compared to the 5% catalyst composition was observed on this catalyst composition.
Thus, the production of a number of products with high yields: acetaldehyde – at 673-723 K, acetone – at 823 K, methanol – at 673-723 K, MEK – at 773-823 K, ethanol – at 823 K, ethylene – at 673- 723 K, H2 – at 823 K is possible during the oxidative conversion of propane-butane mixture at GHSV = 450 h-1 on 1-10% MoCrGa catalysts. It was determined that 1% MoCrGa/TWS catalyst is more suitable for the synthesis of gaseous products. It has been established that 5% MoCrGa catalyst is an optimal for obtaining of high yields as liquid products of partial oxidation and oxidative dehydrogenation products (33.3% acetaldehyde, 50.9% acetone, 15.7% methanol, 37.6% MEK, 28.3% ethylene and 18.8% H2).
The polyoxide MoCrGa catalysts supported on natural clays investigated in the oxidative conversion of propane-butane mixture were studied by physical-chemical methods. The specific surface and porosity of the studied sorbent samples were determined by the BET method for low-temperature nitrogen adsorption. It was established that the clay surface is 10-16 m2/g, and the change in the optimum pore radius was observed from 20 to 50 Å. Elemental analysis of the initial and processed samples of sorbents with 10% HCl showed that the oxide compounds of Si, Al, as well as Ca, Mg, Fe, and Na are predominantly present in clay samples. The ratio of SiO2/Al2O3 (silicon module) was 5-0.4. The silicon module increased after acid treatment, but the phase composition remained practically unchanged. In the course of work, it was determined that the yields of target products on the catalysts pretreated with 10% HCl exceed analogous yields on the untreated catalysts. It is assumed that acid treatment of sorbents contributed to development of surface and increase in the pore radius, which led to increase in the yield of target products of the oxidative conversion of propane-butane mixture.
It was established by the XRD method that the kaolinite Al2[OH]4Si2O5 (JCPDS-29-1488), a-quartz SiO2 (JCPDS 5-490) and the X-ray amorphous component (short-range order 4.20 Å) are the main phase of natural clay. The diffractograms of spent catalysts under reaction conditions at 573 and 773 K are identical. The 3.62, 2.66, and 2.48 reflexes, relating to the phase of Cr2O3 (JCPDS 6-504) and the 3.52, 2.67, 2.38 reflexes, relating to the phase of Cr3O12 (JCPDS 18-390) were detected. The 3.52 reflex refers, perhaps, to textured kaolinite. The structural elements relating to Ga and Mo were not detected because of their dispersity.
Electron microscopic studies have shown that the presence of a large number of insoluble components, which make it difficult to decipher the deposited phases is characteristic for carrier. Large particles and aggregates from large dense particles are characteristic for the initial samples of MoCrGa. Their microdiffraction pattern is represented by separate rare reflections attributed to Cr2O3 (JCPDS, 6-508) and CrO (JCPDS, 6-532), as well as to semitransparent plate-like particles, the micro diffraction pattern from which is represented by reflexes located on a hexagonal motif referred to CrMoO4 (JCPDS, 34-474). The presence of combined chromium-molybdenum-gallium phases is characteristic for samples processed under experimental conditions.
Figure 4a shows small clusters composed by particles with a size of 30-50 Ǻ and big plate-like particles. A mixture of rings and separate reflexes presents the microdiffraction. The rings correspond to CrMoO4 phase (JCPDS, 29-452) – dispersed particles. The big plate crystals correspond to CrMoO6 (JCPDS, 33-401).
Figure 4b shows an aggregate of dense particles with signs of cutting with a minimum dimension of ~ 200°C. The microdiffraction pattern is presented by reflexes arranged along the rings, and corresponds to a mixtures of Cr0.17Mo0.83 =O2 (JCPDS, 34-473) and CrO (JCPDS, 6-532).
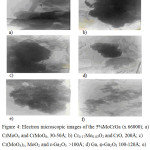 |
Figure 4: Electron microscopic images of the 5%MoCrGa (х 66000); a) CrMoO4 and CrMoO6, 30-50Å; b) Cr0.17Mo0.83O2 and CrO, 200Å; c) Cr(MoO4)3, MoO2 and ε-Ga2O3 ˃100Å; d) Ga, φ-Ga2O3 100-120Å; e) ε-Ga2O3 ~50Å; f) Cr2O5 ~30Å. |
The microdiffraction pattern of aggregate (Figure 4c) is presented by rings and reflexes which are located by series and corresponds to a mixture of Cr(MoO4)3 (JCPDS, 20-309), MoO2 (JCPDS, 9-209) and, possibly, ε-Ga2O3 (JCPDS, 6-503) phases, where there are aggregates with a size more than 100 Ǻ and individual large particles. A small aggregate (Figure 4d) with a particle size of 100-120 Ǻ is presented in a microdiffraction pattern by separate reflexes and corresponds to φ-Ga2O3 (JCPDS, 20-426) in a mixture with Ga (JCPDS, 31-539). The extensive aggregation (Figure 4e) from dispersed particles of size ~ 50Ǻ corresponds to ε-Ga2O3 (JCPDS, 6-509). More small dispersed particles (Figure 4f) size of ~ 30Ǻ are assigned to Cr2O5 (JCPDS, 36-1329).
Comparison EM images of the 1-10% MoCrGa samples showed that the set of phases is significantly reduced at simultaneous enlargement of particles with increasing the content of active component on carrier.
CrMoO2 + CrO spinels with a particle size of 600 Å and Ga2O3 of various modifications (α and φ) as well as Cr5+ remain on the surface of the 5% MoCrGa sample treated at 623 K. The Cr5+, CrOOH phases disappear on the surface. The phases corresponding to Ga3+ and Cr3+ are present in all catalysts, becoming somewhat larger in size. The appearance of spinel (CrMoO6) with a size of 500 Å, which was absent at 573 K, and also Cr2+ is a distinguishing feature of the highly active optimal 5% MoCrGa catalyst heated at 823 K. This facilitates the process both towards partial oxidation and towards oxidative dehydrogenation with optimum production of the desired reaction products.
Conclusion
Thus, developed three-component catalytic systems based on Mo, Cr and Ga showed catalytic activity in gas-phase and liquid-phase oxidation of linear hydrocarbons to aldehydes, alcohols, ketones and olefins. The research of oxidative conversion of propane-butane mixture on polyoxide catalysts based on molybdenum, chromium and gallium supported on natural clay allowed to determine that preferential composition of products is determined by temperature of process. It was found that 5% MoCrGa catalyst in which by varying the reaction temperature was obtained up to 33% acetaldehyde, 50.9% acetone, 38% MEK, 15.7% methanol, 28.3% ethylene and 18.8% hydrogen is most active when the content of active phase of catalyst varies from 1 to 10% on a carrier.
Acknowledgments
The work was supported by the Ministry of Education and Science of the Republic of Kazakhstan (АР01133881, BR05236739).
Conflict of Interest
There is no conflict of interest.
References
- Liu, G.; Zhao, Z. J.; Wu, T. F.; Zeng, L.; Gong, J.L. ACS Catal. 2016, 6, 5207-5214.
CrossRef - Im, Y.; Lee, J.; Kwak, B.; Do, J.; Kang, M. Catal. Today. 2018, 303, 168-176.
CrossRef - Peymani, M.; Alavi, S. M.; Rezaei, M. Int. J. Hydrogen Energy., 2016, 411, 19057-19069.
CrossRef - Wei, C.; Luo, J.; Paul, S.; Liu, Y.; Khodakov, A.; Bordes, E. Catal. Today. 2017, 298, 145-157.
CrossRef - Mitran, G.; Ahmed, R.; Iro, E.; Hajimirzaee, S.; Hodgson, S.; Urda, A.; Marcu, I.-C. Catal. Today. 2018, 306, 260-267.
CrossRef - Arutyunov, V.; Pogosyan, N.; Pogosyan, M.; Tavadyan, L.; Shapovalova, O.; Strekova, L. Chem. Eng. J. 2017, 329, 231-237.
CrossRef - Lodeng, R.; Lunder, O.; Lein, J.E.; Dahl, P.I.; Svenum, I.-H. Catal. Today. 2018, 299, 47-59.
CrossRef - Tu, X.; Niwa, M.; Arano, A.; Kimata, Y.; Okazaki, E.; Nomura, S. Appl. Catal., A. 2018, 549,152-160.
CrossRef - Xu, A.; Wang, Y.; Ge, H.; Chen, S.; Li, Y.; Lu, W. Chinese J. Catal. 2013, 34, 2183-2191.
CrossRef - Tungatarova, S. A.; Baizhumanova, T. S.; Zheksenbaeva, Z. T.; Kassymkan, K. Chem. Eng. Trans. 2017, 61, 1135-1140.
- Baizhumanova, T. S.; Tungatarova, S. A.; Zheksenbaeva, Z. T.; Kassymkan, K.; Zhumabek, M. Chem. Eng. Trans. 2015, 45, 1063-1068.
- Hognon, C.; Simon, Y.; Marquaire, P.M.; Courson, C.; Kiennemann, A. Chem. Eng. Sci. 2018, 181, 46-57.
CrossRef - Urasaki, K.; Kado, S.; Kiryu, A.; Imagawa, K.; Tomishige, K.; Horn, R.; Suehiro, Y. Catal. Today. 2018, 299, 219-228.
CrossRef - Peymani, M.; Alavi, S. M.; Rezaei, M. Appl. Catal., A. 2017, 529,1-9.
CrossRef - Tungatarova, S. A.; Zheksenbaeva, Z. T.; Baizhumanova, T. S.; Grigoriyeva, V. P.; Sarsenova, R. O; Chem. Eng. Trans. 2017, 61, 1915-1920.

This work is licensed under a Creative Commons Attribution 4.0 International License.









