Acute-lethal Toxicity (LC50) Effect of Terminalia Catappa Linn. Leaves Extract on Oreochromis Niloticus (Red Nile Tilapia) Juveniles Under Static Toxicity Exposure
Kamaruzzaman Yunus*1 , Ainatul Mardhiah Jaafar1 and Akbar John2
, Ainatul Mardhiah Jaafar1 and Akbar John2
1Department of Marine Science, Kulliyyah of Science, International Islamic University Malaysia (Kuantan Campus), Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200, Kuantan, Malaysia.
2Institute of Oceanography and Maritime Studies (INOCEM), Kulliyyah of Science, International Islamic University Malaysia (Kuantan Campus), Jalan Sultan Ahmad Shah, Bandar Indera Mahkota, 25200, Kuantan, Malaysia.
Corresponding Author E-mail: kama@iium.edu.my
DOI : http://dx.doi.org/10.13005/ojc/350132
Article Received on : 20-09-2018
Article Accepted on : 14-02-2019
Article Published : 25 Feb 2019
Presnt study was conducted to assess the toxic effect of Terminalia catappa leaf extract on the red Nile tilapia (Oreochromis niloticus) juveniles under static toxicity exposure (96 h exposure). Different concentration of leaf extracts was administered (700 ppm, 800 ppm, 900 ppm, 1000 ppm and 1100 ppm) on red tilapia juveniles in 5 experimental tanks in triplicate. A total of 10 red Nile tilapia juveniles per tank were used as experimental specimens. Median lethal concentration value, LC50 was determined and juvenile behavioral changes were documented. Results showed that the LC50 value was 900 ppm at a random range of time up to 96 h. Present study will be beneficial in considering the proper utilization of T. catappa leaf as potential antibacterial agent in conventional aquaculture practices.
KEYWORDS:Juvenile; Oreochromis Niloticus; LC50; Terminalia Catappa; Toxicity Test
Download this article as:| Copy the following to cite this article: Yunus K, Jaafar A. M, John A. Acute-lethal Toxicity (LC50) Effect of Terminalia Catappa Linn. Leaves Extract on Oreochromis Niloticus (Red Nile Tilapia) Juveniles Under Static Toxicity Exposure. Orient J Chem 2019;35(1). |
| Copy the following to cite this URL: Yunus K, Jaafar A. M, John A. Acute-lethal Toxicity (LC50) Effect of Terminalia Catappa Linn. Leaves Extract on Oreochromis Niloticus (Red Nile Tilapia) Juveniles Under Static Toxicity Exposure. Orient J Chem 2019;35(1). Available from: https://bit.ly/2BPXQwC |
Introduction
The acute toxicity test is one of the alternative methods to figure out the effect of toxicant to a particular tested organism. Study of acute toxicity is a platform for fast and informative method.1 The test is an advantage as the outcome can be obtained in the shortest period of 96 hours. It is a common practice in piscicide bio-safety assessment to identify the toxicity level of a substance to fisheries, prior to be applied in sustainable aquaculture industries. Bio-safety in aquaculture, fish feed is crucial for human consumption, especially for public health concern in developing countries.2,3 The fish feed needs to fulfill the regulatory compliance, prior to be marketed as a domestic food source.2,4,5 Thus, the result outcome is expected to be safe for the aquatic species and for the human intake. Other than that, the test will assist in evaluating the possible risk to the similar fish species in natural environments. Acute toxicity test also is possible to be tested on other fish species as comparative purpose.6
The properties of synthetic piscicide that is non-biodegradable is possible to alter the marine ecosystem and caused hazardous water pollution.7 Hence, the utilization of natural products has gained the attention of aquaculture farming industries, as it is ecologically safe and harmless for consumer.8 Piscicide of plant origin, is generally preferable as the effect can be aimed for not affecting the aquatic species of interest and not involving chemical substance. Besides, eco-toxic properties of plant origin piscicides have become another interest for consumers.8,9
Practices of medicinal plants as an antimicrobial is one of the preferred selections by the fish farmers.10 The properties of medicinal plants promote the growth and tonic, accordingly improve the immune system.11 Studies about the properties of medicinal plant component was conducted since late 19th century and practice of drugs that originally come from plant have been widely known. Medicinal plants are originally from organic compound and considered as natural products.9,12 The application of medicinal plants is potential remedies in fighting against fish disease, boosters of stress resistance and good defense from any infections.13 Phytochemical elements such as phenolic, polyphenols, alkaloids, quinones, terpenoids, lectins and polypeptides contents were figured out in medicinal plants. These elements are potential as an alternative to antibiotics and other synthetic compounds.11 Pandey et al., (2012) stated that other phytochemical elements such as tannins, alkaloids and flavonoid, are the factors of the medicinal plant to be resisted against diseases 13. Besides, medicinal plants have various advantages due to the lower price, minimum side-effects, and environmental friendly compared to synthetic piscicide.11,13,14
However, the concentration of the plant extract needs to be evaluated as concentrated extract may cause mortalities to the non-target aquatic species.15 It is important to analyse the antimicrobial of plant origin, prior to usage. This is crucial to prevent the utilization of the plant origin antimicrobial that may affect the non-targeted fish species.8 Hence, the aquatic toxicity test of median lethal concentration or also known as LC50 is crucial to be applied in order to evaluate the performance of any antimicrobial. LC50 is known as a standard measure of the toxicity that will kill half of the population. LC50 can be a tool to set the safe concentrations of pollutant in our environment based on eco-toxicological perspectives and become part of parameters that relate to acute lethal toxicity of pollutants.
Terminalia catappa have been used in aquaculture practice to combat pathogenic microbes and to enhance the microbial resistance in the target species under culture. It is also called as Indian almond, Bengal almond, Singapore almond, Malabar almond, Talisay almond or Tropical almond. This plant can be found throughout the tropics in coastal environments.16 Parts of T. catappa such as leaves, fruits, and bark have been used for years to treat disease as it has high antioxidant and anti-microbial properties. It was applied to all living things, including fishes to combat a certain disease that is caused by fish ectoparasite, fungi or bacteria.17 However, only less than 10% of herbal products is allowed for consumption as an overdose of herbal concentration may cause fatal to the consumers.15
It was known that T. catappa is potential to improve the environment to betterment. It was reported that the extraction of T. catappa can reduce the pH of water-system and the toxicity.18 Indirectly, it also contributes nutrients and functioned as anti-bacterial defense, especially in an ornamental fish culture.19 T. catappa has wound healing properties and trigger spawning. It is used to treat injured Siamese fighting fish as it can promote substance for wound healing.19,20 The antiparasitic, antibacterial and antifungal activities derived from a T. catappa solution was reported to be excellent against some red Nile tilapia pathogens.17 Hence, it is suggested that the studies about the additional toxicological of T. catappa should be further studies to ensure the herbal extract is safe to use.
In this study, Red Nile tilapia (Oreochromis niloticus) juvenile fishes were tested with T. catappa. Red Nile tilapia fish is a common fish species inland waters and brackish water regions. The physical characteristics of red Nile tilapia includes its shaped like a sunfish or crappie. However, it can easily recognize by an interrupted characteristic of the lateral line of the Cichlid family of fishes. Besides, red Nile tilapia have deep bodies with long dorsal fins. Red Nile tilapia is one of a robust and most cultured species in aquaculture farming. Over 800,000 metric tons of red Nile tilapia is harvested and regard as widely freshwater aquaculture in the world.21 Red Nile tilapia can withstand a wide range of environmental stress and disease conflict such as high temperature, salinity, low dissolved oxygen content and high content of ammonia concentrations. Besides, they have valuable properties such as fast growing and consume an enormous range of natural food organism.22,23 Hence, it has become the model fish-species for toxicological studies in Hong Kong and Southeast Asian countries. A study of acute toxic effects of deltamethrin on red Nile tilapia, O. niloticus was conducted by Boateng et al., (2006).24 In another study by Ayoola (2008), the group conducted a study regarding the toxicity of glyphosate herbicide on O. niloticus juveniles. Juveniles have been used due to its economic importance and ease of handling for laboratory scale.25
The production of red Nile tilapia (Oreochromis niloticus) is expected to be enhanced by applying T. catappa leaves extract as the piscicide. Therefore, toxicological assessment needs to be conducted to identify the acceptable concentration of T. catappa that is safe to be used. There is limited study about the toxicity of T. catappa on red Nile tilapia juveniles. Most of the previous studies just related with the properties and benefits of T. catappa. Hence, this study was conducted to evaluate and determine the toxicity level of T. catappa towards red Nile tilapia juveniles via acute toxicity test of median lethal concentration, LC50.
Materials and Methods
Preparation of Terminalia Catappa Extract
The leaves of T. catappa was collected along the coastal area of Pantai Sepat, Pahang seaside. The leaves were then rinsed in flowing water to eliminate sand and dried in an oven with the temperature range of 45-55°C. The dried leaves were powdered by an electronic food grinder and sieved using sieve-shaker with <63 micron (μ) sieve. The fine powder of T. catappa leaves were mixed and diluted with distilled water to make pure stock solution according to the method described by Tasneem et al., (2014).26
Preparation of Red Nile Tilapia (Oreochromis Niloticus) Culture
Red Nile tilapia (O. niloticus) juveniles with size range between 1 inch to 1 ½ inches were purchased from the SAG Aqua Group Company. All the fishes were transported in large concrete holding tanks to Institute of Oceanography and Maritime Studies (INOCEM). Red Nile tilapia is acclimatized for two weeks, prior to further experiment. This is to provide adaptation for the red Nile tilapia fishes, which freshly purchased. Continuous aeration was provided to all the red Nile tilapia. The red Nile tilapia is fed twice daily to satiation with commercial feed contained 30% of crude protein.
Acute-Lethal Toxicity Test, LC50
A total of 5 tanks (in triplicate) were tested with different concentrations and an additional tank was acting as a control. Each 5-L tank contained ten red Nile tilapia juveniles. The concentration of T. catappa that was used on red Nile tilapia juveniles were 700 ppm (parts per million), 800 ppm, 900 ppm, 1000 ppm and 1100 ppm. Static toxicity exposure was applied in the study. The solution was remaining unchanged along the period of the study. Water parameters such as pH, dissolved oxygen and temperature were recorded before and after the experiment. Any mortality and abnormal behavior of red Nile tilapia were recorded along the 96 h. Death was defined as complete immobility with no flexion of the abdomen upon forced extensions.27
Results and Discussion
Red Nile tilapia juveniles that were tested with higher concentration of T. catappa solution becomes less active and sometimes hyperactive compared to the fish in the control tank. The exposure exhibit to changes in opercula rate and may cause physical damages to fish, particularly on the gill surfaces 28. Table 1 shows the percentage of red Nile tilapia mortality (%) in different concentration of T. catappa according to time intervals. The numbers of surviving fish were reduced for some tank when the exposure time increased. Most concentrated tank which is 1100 ppm of T. catappa showed an early toxicity effect during the first 2 h of the experiment, thus indicated the fastest red Nile tilapia mortalities time compared to others. The tank with the lowest concentration of T. catappa (700 ppm), the first lethal time of fish only can be identified at 12 h of the experiment. This shows that the toxic effect of T. catappa towards fish was faster at concentrated solution compared to a less concentrated solution. The toxicity of T. catappa significantly reacted with fish during the first 36 h of the test. After that time, there was no more mortality of fish recorded. This might due to no more exposure to toxicity effect toward the fish after a certain period, thus the fish has gained tolerance towards the concentration.
Table 1: Percentage of red nile tilapia mortality (%) in different concentration of T. catappa according to random time intervals.
| Hour | Percentage of red nile tilapia mortality (%) in different concentration of T. catappa | |||||
| Blank | 700 | 800 | 900 | 1000 | 1100 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 10 |
| 4 | 0 | 0 | 10 | 10 | 20 | 30 |
| 8 | 0 | 0 | 0 | 10 | 10 | 20 |
| 12 | 0 | 10 | 0 | 20 | 10 | 10 |
| 16 | 0 | 10 | 10 | 0 | 0 | 10 |
| 24 | 0 | 0 | 10 | 0 | 10 | 10 |
| 36 | 0 | 0 | 0 | 10 | 10 | 0 |
| 48 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 | 0 | 0 | 0 | 0 | 0 | 0 |
| 62 | 0 | 0 | 0 | 0 | 0 | 0 |
| 72 | 0 | 0 | 0 | 0 | 0 | 0 |
| 96 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total mortality of red Nile tilapia (%) | 0 | 20 | 30 | 50 | 60 | 90 |
The percentage of fish mortality can be referred in Table 2. It showed that the tank with the highest concentration of T. catappa solution (1100 ppm) recorded the highest mortalities of fish with 90% of the fish died. Tank with minimum T. catappa solution which is only 700 ppm recorded the lowest percentage of fish mortality compared to other tanks. The lethal concentration of T. catappa towards red Nile tilapia juveniles was identified in this test. Fifty percent (50%) of red Nile tilapia fish mortalities successfully recorded in a tank with concentration of 900 ppm. This remark the LC50 value of this toxicity test. The result showed that, 900 ppm for T. catappa concentration is the safety boundaries of the T. catappa. Hence, it is secure to use T. catappa as long as it is not exceeding the concentration of 900 ppm.
Table 2: Percentage of fish mortality (%) in different T. catappa concentration (ppm).
| Concentration of T. catappa (ppm) | No. of dead red Nile tilapia juveniles | Percentage of mortality (%) |
| 0 (Control) | 0 | 0 |
| 700 | 2 | 20 |
| 800 | 3 | 30 |
| 900 | 5 | 50 |
| 1000 | 6 | 60 |
| 1100 | 9 | 90 |
The LC50 results (Figure 1) that was obtained enable the practical identification of the occurrence of contamination intensity in the habitat of the red Nile tilapia. If the outcome submitted to a level of contamination higher than the concentration obtained by LC50, it shows higher risk of fishes not to survive in the environment.
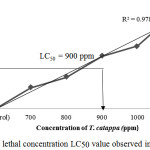 |
Figure 1: Median lethal concentration LC50 value observed in this experiment. |
The qualities of the water in the tested tanks can be evaluated in Table 3. No much changes were shown in the temperature of all tanks. However, dissolve oxygen content parameter shows some changes, yet still in the acceptable range. However, changes of pH value were observed in the study. The pH value was reduced to acidic, which is expected as T. catappa is known to lower the pH of water.18 The decreasing pH value of water in the tested tank from alkali to acidic caused the unfavorable condition to red Nile tilapia, thus lead to mortalities.
Table 3: Water quality parameters in each treatment tanks.
|
Temperature (°C) |
pH |
Dissolved Oxygen (mg/L) |
|||||
| Before | After | Before After Before | After | ||||
| Blank 28.34 28.65 | 7.38 7.55 5.81 | 6.12 | |||||
| 700 ppm | 28.27 | 28.41 | 7.21 | 7.03 | 5.99 | 6.13 | |
| 800 ppm | 28.33 | 28.79 | 7.39 | 6.99 | 5.56 | 6.24 | |
| 900 ppm | 28.45 | 28.56 | 7.15 | 6.23 | 5.30 | 6.44 | |
| 1000 ppm | 28.62 | 28.15 | 7.70 | 6.34 | 5.77 | 6.28 | |
| 1100 ppm | 28.41 | 28.68 | 7.37 | 6.11 | 5.65 | 6.33 | |
Conclusion
In conclusion, the acute toxicity test of T. catappa on red nile tilapia juveniles showed LC50 value of 900 ppm. The response by tilapia juvenile against T. catappa leaf extract towards was observed during first 36 h. This study showed that higher concentration of T. catappa caused higher mortality of red Nile tilapia juveniles. Efforts are needed to determine the acceptable exposure level of T. catappa leaf in aquaculture practices to address the knowledge gap.
Acknowledgements
The research work was sponsored under Research Postdoctoral grant scheme (RPDF 18-004-0004) IIUM.
References
- Syngai, G. G.; Dey, S.; Bharali, R. Asian. J. Pharm. Clin. Res. 2016, 9, 417-421.
- Adesina, B. T. Toxicity of Moringa oleifera (Lam.) extract to Oreochromis niloticus fingerlings and juveniles. University of Ibadan, 2008.
- Adeyemo, O. Food safety and environmental health concerns: Threats to sustainable aquaculture development in Nigeria. In World Aquaculture Conference, 2012.
- Food and Agriculture Organization (FAO). Yearbook of Fishery Statistics, 2000. Accessed online:http//www.fao.org on 14th Feb, 2004.
- Faturoti, E. O. Beneath the Ripples and Sustainable Fish Production. University of Ibadan, 2000.
- USEPA – United State Environmental Protection Agency. Methods for measuring the acute toxicity of effluents to freshwater and marine organisms.4th Ed. Environmental Monitoring and Support Laboratory, US, United State Environmental Protection Agency, Cincinati, Ohio, 2000, EPA600/4-85/013.
- Mian, L. S.; Mulla, M. S. J. Agr. Entomol. 1992, 9, 73-98.
- Singh, D.; Singh, A. Chemosphere. 2002, 49, 45-49.
CrossRef - Son, C. R. I. M.; Mohiseni, M. MAPP. 2017, 1, 1-5.
CrossRef - Direkbusarakom, S. WJST. 2011, 1, 7-14.
- Citarasu, T. Aquac. Int. 2010, 18, 403–414.
CrossRef - Chanda, S.; Nair, R. Indian. J. Pharmacol. 2006, 38, 142-144.
CrossRef - Pandey, G.; Sharma, M.; Mandloi, A. K. Plant Archives. 2012, 12, 1-4.
- Azrul, L. M.; Nurulaini, R.; Adzemi, M. A.; Marina, H.; Effendy, A. W. M. J. Nat. Prod 2014, 7, 98-103.
- Ifeoma, O.; Oluwakanyinsol, S. Screening of Herbal Medicines for Potential Toxicities. In New Insights into Toxicity and Drug Testing; Gowder, S., Ed.; IntechOpen: Croatia, 2013.
CrossRef - Mohale, D.; Dewani, A. Journal of Herbal Medicine and Toxicology. 2009, 3, 7-11
- Chitmanat, C.; Tongdonmuan, K.; Khanom, P.; Pachontis, P.; Nunsong, W. Acta Hortic. 2005, 678, 179–182.
CrossRef - Nagi, A.; Abdullah, M. I.; Jasmani, S.; Zakariah, M. I.; Karim, U.; Hassan, M. Res. J. Fish. Hydrobiol. 2016, 11, 1-6.
- Nugroho, R. A.; Manurung, H.; Saraswati, D.; Ladyescha, D.; Nur, F. M. Biosaintifika: Journal of Biology & Biology Education. 2016, 8(2), 240-247.
CrossRef - Chansue, N.; Assawawongkasem, N. KKU Vet. 2008, 18, 36-45.
- Popma, T.; Masser, M. SRAC Publication No. 283 1999, 1-4.
- Taweel, A.; Shuhaimi-Othman, M.; Ahmad, A. K. Ecotoxicol Environ Saf. 2013, 93, 45-51.
CrossRef - Ogunbona, A. A.; Ijimakinde, B. J. Fish. Aquat. Sci. 2014, 9, 330-337.
CrossRef - Boateng, J. O.; Nunoo, F. K. E.; Dankwa, H. R.; Ocran, M. H. West African Journal of Applied Ecology. 2006, 9, 5-9.
- Ayoola, S. O. Afr. J. Agr. Res. 2008, 3, 825-834.
- Tasneem, S.; Kauser, S. H.; Yasmeen, R. J. Biopest. 2014, 7, 124-131.
- Lockwood, A. P. M. Effects of Pollutants on Aquatic Organisms. – Society for Experimental Biology, Seminar Series 2.; Cambridge University Press: Cambridge‐London‐New York‐Melbourne, 1976.
- Roberts, R. J. Fish pathology; John Wiley & Sons: Scotland, 2012.

This work is licensed under a Creative Commons Attribution 4.0 International License.









