Triton-B Mediated One-Pot Multicomponent Synthesis of 3,5 Substituted Tetrahydro-2H-1,3,5-Thidiazine-2-Thiones
Ram Kishore1, Monika Kamboj1 and Manisha Shukla2
and Manisha Shukla2
1Department of Applied Chemistry, Amity School of Applied Sciences, Amity University Uttar Pradesh, Lucknow Campus, Lucknow-226028, U. P, India.
2Department of Chemistry, BBDNITM, Lucknow-227105, U. P, India.
Corresponding Author E-mail: mkamboj@lko.amity.edu
DOI : http://dx.doi.org/10.13005/ojc/340627
Article Received on : 30-06-2018
Article Accepted on : 27-09-2018
Article Published : 02 Nov 2018
A new-fangled, proficient, one-pot multi component, intramolecular C-S bond formation reaction, mediated by phase transfer catalyst, Triton-B, is described in this paper. The reaction of alkyl/ phenyl amines, CS2 and formaldehyde catalyzed via Triton-B resulted in formation of 3-(Alkyl or aryl methyl),5-(Alkyl or aryl methyl) substituted tetrahydro-2H-1,3,5-thiadiazine-2-thiones compounds(1a-15a). These compounds (1a-15a) were characterized with the help of elemental analysis, IR, NMR and mass spectroscopic methods. The PTC mediated reactions require mild reaction condition and reduced time period for completion. The reaction is achieved at normal temperature under solvent free conditions with good yields and great selectivity. This methodology discourages the traditional synthesis method of inorganic base for such coupling reaction.
KEYWORDS:CS2; Multi Component; Phase Transfer Catalyst; Triton-B
Download this article as:| Copy the following to cite this article: Kishore R, Kamboj M, Shukla M. Triton-B Mediated One-Pot Multicomponent Synthesis of 3,5 Substituted Tetrahydro-2H-1,3,5-Thidiazine-2-Thiones. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Kishore R, Kamboj M, Shukla M. Triton-B Mediated One-Pot Multicomponent Synthesis of 3,5 Substituted Tetrahydro-2H-1,3,5-Thidiazine-2-Thiones. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=51900 |
Introduction
3,5 substituted tetrahydro-2H-1,3,5-thiadiazine-2-thiones (THTT), an important scaffold have found utility as chemicals for crop protection,1 against soil nematodes,2 antitumor drugs,3 precursors in organic synthesis4 and as antimicrobial agent.5Recently dithiocarbamates developed as a novel class of prospective agrochemicals.6-9 Also they are potential pharmaceutical drugs against microbial infection,10 protozoa,11 leprosy,12 tubercular,13 fungal,14 Leishmaniasis,15 Alzheimer’s disease.16 Owing to ample of application, there is growth of well-planned methods for preparing 3-(Alkyl or aryl methyl),5-(Alkyl or aryl methyl) substituted tetrahydro-2H-1,3,5-thiadiazine-2-thiones. New strategies for the synthesis of 3-(Alkyl or aryl methyl),5-(Alkyl or aryl methyl) substituted tetrahydro-2H-1,3,5-thiadiazine-2-thiones by using Triton-B, a phase transfer catalyst (PTC) delightfully supplement the conventional approach of cyclization by multicomponent reaction,17-18 based on inorganic base reactions. The inorganic base such as NaOH, KOH, Na2CO3, H2O / EtOH, uniform H2O catalyzed intra-molecular cyclization of amines, CS2 and formaldehyde that were produce in situ or resynthesized are the traditional recur methods19-20 (Previous work) “Scheme 1”. On the one hand, current effort is PTC-mediated prolific methodology to prepare THTT (Current work) “Scheme 1”. The adeptness of this method is (i) excess amounts of toxic reagents are avoided in this scheme that adversely affects the health. (ii) cost of PTC is low (iii) high yield (iv) reduced reaction time. (v) Triton-B is recovered from the reaction mixture by filtration.21 These factors discourage the use of inorganic base for such coupling reactions. This simple procedure with mild reaction conditions are safer and more sustainable practices for effective coupling reaction mediated by Trimethylbenzyl ammonium hydroxide (Triton-B) without using any inorganic base,22 Scheme 1.
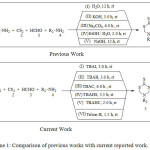 |
Scheme 1: Comparison of previous works with current reported work. |
Materials and Methods
All the chemicals were of AR grade. Reactions were done under an envelope of Ar gas. Bomem MB-104-FTIR spectrophotometer was used for recording Infra Red Spectra in the range 4000-200 cm-1. The AC-400F-NMR spectrometer, with Me4 Si as internal standard was used for recording 1HNMR spectra at 400 MHz. The investigation of elements were conveyed with the help of a Carlo-Erba EA 1110-CNNO-S analyzer. There is good covenant between observed and calculated values.
General Method for the synthesis of compounds (1a-15a) using Triton-B:
At room temperature 1.0 mmol of primary amine (1) and 10.0 mmol CS2 (2) and 2.5 mmol formaldehyde (3) was stirred for 0.25h. Then to this solution, 1.5 mmol Triton-B was added and stirred for 0.25h. Afterward 1.0 mmol amine (4) was added at room temperature for 2h, the stirring of reaction mixture was continued. The advancement of reaction was observed through Thin Layer Chromatography. After accomplishment of reaction, 50 mL water was added and thrice treated with CH3COOC2H5 (20 mL each). The crude raw product was obtained by concentrating ether layer under low pressure which was further refined by silica gel (100-200 mesh) column chromatography by using eluent 50% (EtOAc : Hexane) to afford pure product.
Results and Discussion
In the present work, series of substituted THTT (1a-15a) are synthesized by Triton B mediated one-pot multicomponent reaction, a novel protocol (Scheme 2). Earlier THTT was produced from amines, CS2 , formaldehyde using inorganic base.23-25 A number of PTC is frequently used for the synthesis of THTT.26 On comparing the reaction condition it was comprehended that by using Triton-B, % yields of preferred product is improved than other types of PTC.17 Triton-B give 95% yield of THTT
A range of 1o amines (aliphatic, alicyclic, heterocyclic, aromatic) and formaldehyde using Triton-B/CS2 method at r.t led to formation of product THTT in excellent yields, Table 1. It has been observed that electron donating group in R1NH2 or R2NH2 gives corresponding products in good amount. These compounds were characterized by spectroscopic techniques (Fig 1-3). Proposed reaction and Mechanisms shown in Scheme-2.
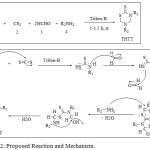 |
Scheme 2: Proposed Reaction and Mechanism. |
Table 1: Effect of Substituents on THTT formation.
| Compound | R1 | R2 | Molecular Formula | Time (h) | Yield % |
| 1a | C2H5 | C2H5 | C7H14N2S2 | 1 | 95 |
| 2a | 4-Cl-PhCH2 | 4-Cl-PhCH2 | C17H16Cl2N2S2 | 1 | 94 |
| 3a | C2H5 | 2-MeO-PhCH2 | C13H18ON2S2 | 1 | 92 |
| 4a | C7H15 | 3-CF3-PhCH2 | C18H25F3N2S2 | 1 | 94 |
| 5a | Ph | Ph | C15H14N2S2 | 1.5 | 95 |
| 6a | 3-Cl-PhCH2 | 4-Cl-PhCH2 | C17H16Cl2N2S2 | 1 | 93 |
| 7a | 2-F-PhCH2 | 2-F-PhCH2 | C17H16F2N2S2 | 1 | 93 |
| 8a | PhC4H8 | 4-CF3-PhCH2 | C21H23F3N2S2 | 1 | 90 |
| 9a | C2H5 | 2-F-PhCH2 | C12H15FN2S2 | 1 | 94 |
| 10a | 2-F-PhCH2 | 4-Cl-PhCH2 | C17H16ClFN2S2 | 1 | 90 |
| 11a | C2H5 | C4H9O | C9H18ON2S2 | 1.5 | 92 |
| 12a | PhC4H8 | PhC4H8 | C23H30N2S2 | 1.5 | 93 |
| 13a | C5H11 | C5H11 | C13H26N2S2 | 1.5 | 90 |
| 14a | C4H9 | PhC4H8 | C17H26N2S2 | 1.5 | 91 |
| 15a | C7H15 | C7H15 | C17H34N2S2 | 1 | 93 |
R1 and R2 =1.0mmol each
Spectral Data of selected synthesized dithiocarbamates (1a, 1b, 1c)
3,5-diethyl-1,3,5-thiadiazinane-2-thione (1a)
Yield 95%; Off white solid, M.P. 104-109oC; 1H NMR 400 MHz (CDCl3): δ 4.407 (s, 2H), 4.346 (s, 2H), 4.028 (q, J = 7.2 Hz, 2H), 2.823 (q, J = 7.2 Hz, 2H), 1.232 (t, J = 7.2 Hz, 3H), 1.155 (t, J = 7.2 Hz, 3H). 13C NMR 400 MHz (CDCl3): δ 11.634, 12.758, 44.474, 46.951, 57.508, 68.998, 191.114. MS (ESI): m/z=191.06 [M]+. Anal. Calculated for C7H14N2S2: 190.06: C, 44.17; H, 7.41; N, 14.72; S, 33.69. Found: C, 43.95; H, 7.17; N, 14.52; S, 33.48%.
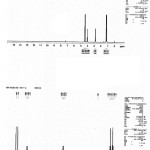 |
Figure 1a: 1HNMR spectra of 3,5-diethyl-1,3,5-thiadiazinane-2-thione. |
 |
Figure 1b: Mass spectra of 3,5-diethyl-1,3,5-thiadiazinane-2-thione. |
3,5-bis(4-chlorobenzyl)-1,3,5-thiadiazinane-2-thione (2a)
Yield 94%; Off white solid, M.P. 105-110oC; 1H NMR 400 MHz (CDCl3): δ 7.364-7.335 (m, 2H), 7.305 (d, J = 8.4 Hz, 2H), 7.236 (d, J = 8.4 Hz, 2H), 7.024 (d, J = 8.4 Hz, 2H), 5.254 (s, 2H), 4.356 (s, 2H), 4.269 (s, 2H), 3.718 (s, 2H). MS (ESI): m/z = 383.09 [M]+. Anal. Calculated for C17H16Cl2N2S2: C, 53.26; H, 4.21; Cl, 18.50; N, 7.31; S, 16.73. Found C, 53.01; H, 4.11; Cl, 18.25; N, 7.11; S, 16.52%.
 |
Figure 2a: 1HNMR spectra of 3,5-bis(4-chlorobenzyl)-1,3,5-thiadiazinane-2-thione. |
3-(2-methoxybenzyl)-5-ethyl-1,3,5-thiadiazinane-2-thione (3a)
Yield 92%; Off white solid, M.P. 106-110oC; 1H NMR 400 MHz (CDCl3): δ 7.521 (d, J = 7.6Hz, 1H), 7.298 (t, J = 6.4 Hz, 1H), 6.952 (t, J = 7.2 Hz, 1H), 6.893 (d, J = 8.0 Hz, 1H), 5.375 (s, 2H), 4.411 (s, 2H), 4.333 (s, 2H), 3.852 (s, 3H), 2.714 (q, J = 7.2 Hz, 2H), 0.943 (t, J = 7.2Hz, 3H). 13C NMR 400 MHz (CDCl3): δ 12.568, 44.521, 48.215, 55.539, 57.915, 68.453, 110.539, 121.072, 123.394, 129.392, 130.369, 157.471, 192.702. MS (ESI): m/z = 282.09 [M]+. Anal. Calculated for C13H18ON2S2: C, 55.29; H, 6.42; N, 9.92; O, 5.67; S, 22.71. Found C, 55.03; H, 6.32; N, 9.82; O, 5.67; S, 22.61%.
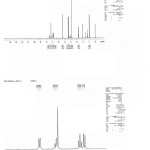 |
Figure 3a: 1HNMR spectra of 3-(2-methoxybenzyl)-5-ethyl-1,3,5-thiadiazinane-2-thione. |
Conclusion
We have developed highly efficient solvent-free one-pot multicomponent coupling of various amines with formaldehyde via CS2/ Benzyl tri-methyl ammonium hydroxide system. This procedure gives corresponding products in good and excellent yield. Also, this procedure has mild reaction conditions, requires shorten time period and has environmental acceptability. This synthetic route thereby offers a more convenient approach for formation of C-S bonds.
Acknowlegements
Ram Kishore and Monika Kamboj appreciative the authorities of Amity University, Lucknow Campus, for their perpetual inspiration.
References
- Lamberth, C. J. Sulphur Chem. 2004, 25, 39-62.
CrossRef - Easton, A. ; Guven, K. ; de Pomerai, D.I. J.Biochem. Mol. Toxicol. 2001, 15, 15-25.
CrossRef - a) Cao, S.L.; Feng, Y.P.; Jiang, Y.Y. ; Liu, S.Y.; Ding, G.Y.; Li, R.T. Bioorg. Med. Chem. Lett. 2005, 15, 1915-1917. (b) Cao, S. L. ; Han, Y. ; Yuan, C.Z. ; Wang, Y. ; Xiahou, Z.K. ; Liao, J. ; Gao, R.T.; Mao, B.B. ; Zhao, B.L. ; Li, Z. F. ; Xu, X. Eur J. Med. Chem. 2013, 64, 401-409.
CrossRef - Hassan, E.A.; Zayed, S.E. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189,300-323.
CrossRef - Ozcelik, A.B. ; Yilmaz, G. ; ozkan, S.; Ersan, S. Rev. Roum. Chim., 2015, 60, 1059-1064
- Haendel, M. A.; Tilton, F.; Bailey, G.S.; Tanguay, R.L. Toxicol. Sci. 2004, 81, 390-400.
CrossRef - Jardim, A.N.O.; Mello, D.C.; Brito, A.P.; Voet, H.V.; Boon, P.E.; Caldas, E.D. Food Chem. Toxicol. 2018, 118, 317-327.
CrossRef - Eng, G.; Song, X.; Duong, Q.; Strickman, D. ; Glass, J. ; May, L. Appl. Organomet. Chem.2003, 17, 218-225.
CrossRef - Rogachev, I.; Kampel, V.; Gusis, V. ; Cohen, N.; Gressel, J.; Warshawsky, A. Pest. Biochem. Physiol. 1998, 60, 133-145.
CrossRef - Guzel, O.; Salman, A. Bioorg. Med. Chem. 2006, 14, 7804-7815.
CrossRef - Ochoa, C.; Pérez, E.; Pérez, R.; Suárez, M.; Ochoa, E.; Rodríguez, H.; Gómez Barrio, A.; Muelas, S.; Nogal, J.J.; Martínez, R.A. Arzneim.-Forsch. 1999, 49,764-769. (b) Coro, J.; Atherton, R.; Little, S.; Wharton, H.; Yardley, V.; Alvarez, A. Jr.; Súarez, M.; Pérez, R.; Rodríguez, H. Alkyl-linked bis-THTT derivatives as potent in vitro trypanocidal agents. Bioorg. Med. Chem. Lett. 2006,16, 1312-1315.
- Marakov, V.; Riabova, O.B.; Yuschenko, A.; Urlyapova, N.; Daudova, A.; Ziplef, P.F.; Mollmann, U. J. Antimicrob. Chemother. 2006, 57, 1134-1138.
CrossRef - Byrne, S.T.; Gu, P.; Zhou, J.; Denkin, S.M.; Chong, C.; Sullivan, D.; Liu, J.O.; Zhang, Y. Antimicrob. Agents Chemother. 2007, 51, 124495-124497.
- Zou, Y.; Yu, S.; Li, R.; Zhao, Q.; Li, X.; Wu, M.; Huang, T.; Chai, X.; Hu, H.; Wu, Q. Euro J. Med. Chem. 2014, 74, 366-374.
CrossRef - Monzote, L.; Montalvo, A.M.; Fonseca, L.; Pérez, R.; Suárez, M.; Rodríguez, H. Arzneim.-Forsch. 2005, 55, 232-8.
- Rehman,A. ; Nafeesa, K. ; Abbasi, M.A. ; Siddiqui, S.Z. ;Rasool, S. ;Shah, S.A.A. ;Ashraf, M. ; Cogent Chemistry, 2018, 4, 1-15.
- Zaidi, S.; Chaturvedi, A.K.; Singh, N.; Chaturvedia, D. Curr. Chem. Lett., 2017, 6, 143-150.
CrossRef - Kishore, R.; Kamboj, M. World j. Pharm. Res., 2018,7, 1098-1109.
- Katiyar, D.; Tiwari, V.K.; Tripathi, R.P.; Srivastava, A.; Chaturvedi, V.; Srivastava, R.; Srivastava, B.S. Bioorg. Med. Chem. 2003, 11, 4369-4375.
CrossRef - Ertan, M. ; Bilgin, A.A.; Palaska, E.; Yulug, N. Arzneim.-Forsch. 1992, 42, 160-163.
- Chaturvedi, D.; Chaturvedi, A.K.; Mishra, N.; Mishra, V. Org. Chem. International. 2012, 2012 ,1- 4.
- Chaturvedi, D.; Zaidi, S. ; Chaturvedi, A.K.;Vaid,S. ;Saxena,A.K. Indian J chem., 2016, 55B 1019-1025
- Ertan, M.; Ayyildiz, H.G.; Yulug, N. Arzneim.-Forsch. 1991, 41,1182-1185.
- Sharma,S. Synthesis, 1978, 11, 803–820.
CrossRef - Pascual, R. M. Synlett, 2015, 26, 1776-1777.
CrossRef - Saeed, S. Cogent chem. 2015, 1,1-10.

This work is licensed under a Creative Commons Attribution 4.0 International License.









