The Function of Cross Linker Carboxylic Acid for TiO2/Chitosan/SiO2 Coated as Self Cleaning Fabrics
Yetria Rilda1, Syukri1, Desi Ferlinda1, Anessa Iasa1 and Anthoni Agustien2
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Andalas University, Indonesia.
2Department of Biology, Faculty of Mathematics and Natural Sciences, Andalas University, Indonesia.
Corresponding Author E-mail: yetriarilda@sci.unand.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340633
Article Received on : 29-10-2018
Article Accepted on : 19-11-2018
Article Published : 19 Nov 2018
The chemical compound TiO2/Chitosan/SiO2 is a photocatalyst with a self- cleaning capability against dye contaminations. Morphologically, it has an anatase structure with 13.1 nm of crystallite size, spherical shape, and Eg = 3.209 eV as characterized by X-ray diffraction, TEM, and UV-DRS. The self-cleaning capability of TiO2/Chitosan/SiO2 against the malachite green dye spot was optimized using two carboxylic acid cross-linkers namely 1,2,3,4- Buthane Tetra Carboxylic Acid (BTCA) and Chloro Acetic Acid (CAA). The optimization in enhanced the growth of ester covalent interaction with the functional groups of textile fiber was performed gradually in CAA and directly in BTCA to produce a self-cleaning textile. The self-cleaning efficiency of cross-linker under the UV irradiation (536 lux for 60 min) was 96% and 69.96% in CAA and BTCA, respectively. The FT-IR analysis revealed that the ester covalent interaction among the cross-linker, cotton fiber, and TiO2/chitosan/SiO2 nanocluster could be determined by the intensity change of C=O stretching of the functional group at 1700-1710 cm-1. The intensity was higher in CAA (15.03%) than BTCA cross-link (12.04%). The superficial morphological feature of the cotton fiber as observed by SEM depicted that TiO2/chitosan/SiO2 particles were dispersed more evenly in the CAA than BTCA. The TiO2/chitosan/SiO2 nanocluster with a CAA cross-link is reliable to be used indesigning a high-quality self-cleaning textile.
KEYWORDS:Cross linker, BTCA, CAA, self-cleaning, Malachite green
Download this article as:| Copy the following to cite this article: Rilda Y, Syukri S, Ferlinda D, Iasa A, Agustien A. The Function of Cross Linker Carboxylic Acid for TiO2/Chitosan/SiO2 Coated as Self Cleaning Fabrics. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Rilda Y, Syukri S, Ferlinda D, Iasa A, Agustien A. The Function of Cross Linker Carboxylic Acid for TiO2/Chitosan/SiO2 Coated as Self Cleaning Fabrics. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52854 |
Introduction
Textile is certainly needed infashion industries as well as various medical and office equipments, andother daily needs. The cotton textile is preferable as compared to the other materials due to its ease of maintenance, the strength, absorption, and affordability.1 However, the higher absorption of cotton fiber makes it easy to be contaminated by dirt and dyes, thus it is also difficult to be removed out.2 As an alternative solution, some researchers modified the textile cotton fiber to acquire the self- cleaning capability against the dye spots contaminating the textile surface. The self- cleaning textile is practically effective to shorten the contact time of the dirt on the textile. Consequently, the dirt could be easily cleaned and the deterioration of the textile fiber could be maximally prevented.3,4
The nano-oxide metals including TiO2, ZnO or its composites are commonly used in modifying the cotton fiberdue to their functional capability as the sunscreen, self- cleaning agents and antimicrobes.3-5 The capabilities of the substances arerelated to their pivotal role asphotocatalysts. Hence, they could remove the dye on the fiber by the photocatalytic mechanism.The previous study by Wijesena, applied this concept to develop the self-cleaning materials by using the TiO2 and ZnO nanoparticles against the dye spots of methyl orange.4 Moreover, Pakdel and Doud used TiO2 and SiO2 nanocomposites to enhance the functional capability of self-cleaning textile against various dyes including methyl orange,4,6,7 methylene blue,3,8 disperse yellow and C.I. reactive red,9 and acid red.10
The coating of nano-oxide metal on the cotton fiber basically requires the cross- link agent, a carboxylic group-consisting compund, in order to promote the covalent interaction with the fibercellulose and TiO2 by means the electrostatics interaction11. The stability of TiO2-SiO2 nanocluster’s coating on the cotton fiber is determined by the type of carboxylic acid used as the cross-linker. A study by Karimi demonstrated that the use ofsuccinate acid as across-linker inthe TiO2-SiO2 particle’s coating could stand its stability up to 85% against washing, while it was only 11% without a cross-linker.5,11,12
Furthermore, our previous study revealed that acrylic acid is an effective cross-linker to developa textile acquiring the antimicrobe and self-cleaning capability against methylene blue dye.4
In this present study, we used two cross-linkers including1,2,3,4 buthane tetra carboxylic acid (BTCA) and chloroacetic acid (CAA) with two different coating methods by meansdirect and gradual coating, respectively. It aimed to enhance the self-cleaning capability against the dye spot of malachite green on a textile cotton fiber with a densely TiO2/chitosan/SiO2 coating.
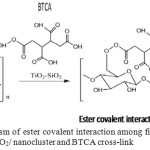 |
Figure 1: Mechanism of ester covalent interaction among fiber cellulose, TiO2/ chitosan/SiO2/ nanocluster and BTCA cross-link. |
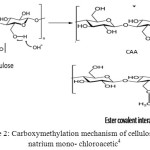 |
Figure 2: Carboxymethylation mechanism of cellulose by natrium mono-chloroacetic4
|
Experimental
Materials
The instruments used in this study were magnetic stirrer, furnace, oven, analytical balance, spin coating machine, optic microscope, SEM-EDX (Hitachi S-3400N), FT-IR (Thermo Scientific: Nicolet iS10), XRD (X’Port PAN Analytical), UV-Vis DRS (Shimadzu UV-Vis 2450), and TEM (JEOL JEM 1400). The materials and chemicals were silky textile cotton, hydrochloric acid (HCl, Merck), acetic acid (CH3COOH, Merck), aquadest, titanium isopropoxide (C12H28O4Ti) (Aldrich 97%), diethanolamine(C4H11NO2) (Merck), isopropanol (C3H8O, Merck), tetra ethyl ortho silicate (C8H20O4Si, Merck), chitosan (C6H11NO4)n, 1,2,3,4-buthane tetra carboxylic acid (C8H10O8, BTCA, Merck), chloroacetic acid (C2H3O2Cl, CAA, Merck), ethyl tri methyl ammonium bromide (C13H33N(CH3)3Br, Merck), detergent, natrium carbonate (Na2CO3),and malachite green (C23H26N2Cl).
Methods
Synthesis of TiO2/chitosan/SiO2 Nano Cluster
The powder of TiO2/chitosan/SiO2 nanocluster was synthesized using a sol-gel method with the titanium isopropoxide (TIP) and tetra ethyl ortho silicate as precursors. The sol solution consisted of A solution (TIP and DEA in isopropanol solvent; 1:2:2 in M) and B solution (TEOS and HCL 2 M in isopropanol; 1:2:2). The A and B solution was homogenized separately for 30 min in RT. The combination of A and B solution was prepared as a mixed sol with a composition of Ti and Si 3:2. Furthermore, the chitosan/acetic acid suspension (1:20) and CTAB (1:5) were added to the sol. The mixed sol was set at pH 10-11 and homogenized for 8 h followed by the heating at 110-120°C for 15 h to produce a gel. The gel was then calcinated at 500°C for 3 h to obtain the powder of TiO2/chitosan/SiO2 nanocluster and subsequently morphologically characterized using XRD, UV-Vis DRS, and TEM.
Coating of Textile Cotton Fiber
The textile cotton of size 64 m2 was firstly washed using detergent 2 g/L and subsequently rinsed with aquadest and dried up at 80°C for 5 min. Furthermore, it was dewaxed using Na2CO3 0.01 g in 25 mL aquadest for 5 min at 100°C and rinsed with aquadest to adjust the pH 6-7, followed by heating at 80°C for 5 min. The textile cotton was directly soaked in 1 M BTCA cross-link for 12 h. In another treatment, the textile was firstly soaked into 2 M NaOH for 30 min then into 1 M CAA. After the treatment using BTCA and CAA, the textile was heated at 80oC for 5 min then coated using a suspension of 1% TiO2/chitosan/SiO2 by means dip-spin coating for 90 min. It was then dried up at 80oC for 15 min and subsequently analyzed using FT-IR, Photo Optics, XRD, and SEM- EDX.
Self-Cleaning Test of the Textile Cotton Fiber
The self-cleaning test was carried outby observing the degradation of the malachite green dye spot on the coated cotton fiber under the UV irradiation (536 lux) in 0-2 h time window. The 4×4 cm textile dropped by 50 μL of 150 ppm malachite green was designated for a qualitative test, while the 1×1 cm textile soaked into 20 mL of 5 g/L malachite green was used for a quantitative test. The textile sample was observed in the darkroom for 30 min and the absorption was subsequently noted as Ao. Furthermore, it was irradiated by UV light in 0-2 h then the absorptionwas noted as A. The dye degradation rate (Ef) was calculated by the formula as follow:
![]()
Results and Discussion
Morphological Characters of TiO2/chitosan/SiO2 Nanocluster
The TiO2/chitosan/SiO2 nanocluster yielded by the sol-gel synthesis method was identified using XRD, UV-Vis DRS, and TEM to determine the morphological characters related to its prospective application as a self-cleaning material. It was found that the nanocluster depicted ananatase structure with 13.1 mm crystallite size at the highest intensity 2θ = 25.3o. The anatase intensity order of TiO2/chitosan/SiO2 was 2θ= 25.3o; 36.93o; and 48o as elucidated by XRD analysis (Figure 3). The crystallite size was calculated by the Scherrer formula as follow:
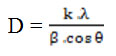
D (crystallite size), k (constant; 0.89), λ (wavelength of X rays), β (value of full width at half maximum, FWHM), and θ (diffraction angle).6,13,14
The crystallite size could be modified by adding the dopant of SiO2 and chitosan. The SiO2 functions to form the pores and to extend the surface area15,16 while the chitosan plays a role as a template of porosity during the condensation of the crystallite and enhances the SiO2 dispersion on TiO2 surface.16 It has been demonstrated that when the TiO2 and SiO2 were composited, it could inhibit the structural transformation of anatase to rutile phase.15 Moreover, we have previously reported that the distribution of crystallite size was affected by the sol and gel preparation process.18 The sol preparation for 8 h and gel polycondensation for 15 h noticeably increased the distributional uniformity of crystallite size of TiO2/chitosan/SiO2 nanocluster. The pattern of UV-DRS spectrum of TiO2/chitosan/SiO2 (presented in Figure 3, left panel was thenconverted using Plank equation.
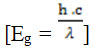
(Figure 3, right panel) with Eg (band gap), h (Plank constant, 6.626 x 10-34 J.s), c (light speed, 3 x 108 m.s-1) and λ (wavelength, in m). It wasrevealed that the maximum wavelength was 386.3 nm and the Eg was 3.209 eV. Hence, the photocatalytic reaction of the TiO2/chitosan/SiO2 could be induced by the UV light (λ= 385 nm) with the energy equals the Eg value.19
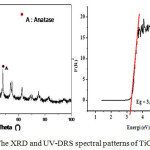 |
Figure 3: The XRD and UV-DRS spectral patterns of TiO2/ chitosan/SiO2 |
The TEM pattern (Figure 4) illustrates the 3-dimensional feature of TiO2 hybridized with SiO2 and chitosan. It was found that the TiO2/chitosan/SiO2 nanocluster has a spherical crystallite shapeand non-agglomerated structure. The SiO2 and chitosan were amorph, and SiO2 and TiO2 formed a core-shell formation with the chitosan functioned as the cross-linker.20 The TEM-derived picture, in consistent with the XRD spectral pattern,indicated that TiO2 has the highest crystallite intensity at 2θ = 25.3o in anatase phase.
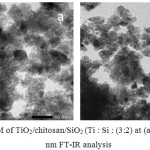 |
Figure 4: TEM of TiO2/chitosan/SiO2 (Ti : Si : (3:2) at (a) 50 nm (b) 100 nm FT-IR analysis.Click here to View figure |
The FT-IR analysis of the cotton fiber coated by TiO2/chitosan/SiO2 with two cross-linkers (CAA and BTCA) produced the FT-IR spectrum as presented in Figure 5. The patterns of the spectrum indicated that the intensity difference was observed at 1710 cm-1 identified as the covalent ester formation’s zone (C=O stretching).21 The CAA cross-link has a higher intensity than BTCA, suggesting that the gradual growing of ester group in CAA could enhance the thickness of TiO2/chitosan/SiO2 coating on the fiber by means the electrostatic interaction.8
The FT-IR spectrum of the uncoated fiber (Figure 5a) depicted its absorbance at cm-1 with the intensity 10.1%, while the coated fiber by CAA cross-link (Figure 5b) indicated the shifted wave number to 1710.04 cm-1 and the increased intensity to 15.03%. Likewise, the coated fiber by BTCA cross-link (Figure 5c) also demonstrated the shifted wave number to 1708.5 cm-1 and increased intensity to 12.4%. The shiftings of the wave number indicated the chemical esterification’ sinteraction between the cellulose of cotton fiber and the carboxylic cross-link as illustrated in Figure 1.
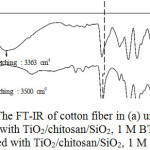 |
Figure 5: The FT-IR of cotton fiber in (a) uncoated (b) coated with TiO2/chitosan/SiO2, 1 M BTCA (c) coated with TiO2/chitosan/SiO2, 1 M CAA. |
The cotton fiber coated by TiO2/chitosan/SiO2 nanocluster with a CAA cross-link depicted the different FT-IR spectrum as compared with BTCA cross-link. Interestingly, the absorbance intensity at 3400 cm-1 and 1529 cm-1 was also observed. The occurrance of the absorbance intensity at 3400 cm-1 might be resulted from the combination of carboxylic absorbance and alchoholic – OH stretchings’s absorbance. The absorbance intensity at 1529 cm-1 was reported as an absorbance of the carboxylic ionic group.4
Morphological Analysis Using SEM-EDX
The morphological observation by using SEM suggested the difference in the features of cotton fiber treated with a CAA cross-link and BTCA cross-link (Figure 6a-c). A cross-link plays a pivotal role in th formation of ester covalent interaction with the hydroxyl group of fiber, there by the coating of the TiO2-SiO2 nanocluster on the textile. Meanwhile, the chitosan in TiO2-SiO2 provides the nitrogen base in order to enhance the amide covalent interaction and thereby trapping the TiO2-SiO2 by means the electrostatic interaction.6 The difference in the cross-link determines the distributional uniformity of the TiO2/chitosan/SiO2 nanoparticle on the textile fiber surface. It was found that in the CAA cross-link-treated fiber, the coating particles dispersed more evenly and densely on the surface of the textile as compared with the BTCA cross-link-treated fiber.
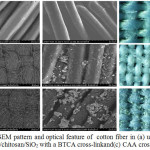 |
Figure 6: The SEM pattern and optical feature of cotton fiber in (a) uncoated and (b) coated by TiO2/chitosan/SiO2 with a BTCA cross-linkand(c) CAA cross-link. |
Chen reported that cotton fiber coated by TiO2 depicted the rough surface feature. The roughness of the surface affects the self-cleaning capability of the fiber.22 The results of SEM analysis (Figure 6) and the pattern of EDX (Figure 7), taken together, suggested that the technique of the growing an ester covalent interaction on the fiber determines the amount of TiO2-SiO2 coating, its distributional uniformity as well as the stability of the coating. Based on the EDX analysis, the composition of C, O, Ti and Si elements was equal to the composition of Ti and Si used as the precursors in the TiO2-SiO2 synthesis (3:2 in M). The elements found in the cotton fiber coated by TiO2/chitosan/SiO2 with a BTCA cross-link consisted of C = 27.6%, O = 17.97%, Ti = 2.36 %, and Si 0.90 %. Otherwise, the elements in the fiber with a CAA cross-link composed of C = 50.73 %, O = 38.64 %, Ti = 2.46 %, and Si 1.45%.
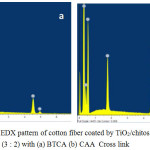 |
Figure 7: The EDX pattern of cotton fiber coated by TiO2/ chitosan/SiO2 (3:2) with (a) BTCA (b) CAA Cross link. |
Self-Cleaning Test
The self-cleaning capability of the cotton fiber coated by TiO2/chitosan/SiO2 nanocluster against malachite green dye spot was evaluated both qualitatively and quantitatively. The qualitative test (Figure 8) revealed that the cotton fiber coated by TiO2/chitosan/SiO2 with a CAA cross-linkacquired the capability to degrade the malachite green dye spot five times stronger as compared with BTCA cross-link. This result is in accordance with the findings by FT-IR and SEM analysis demonstrating that the CAA cross link noticeably elevated the abundance of TiO2/chitosan/SiO2 on the textile fiber.
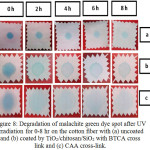 |
Figure 8: Degradation of malachite green dye spot after UV irradiation for 0-8 hr on the cotton fiber with (a) uncoated and (b) coated by TiO2/chitosan/SiO2 with BTCA cross link and (c) CAA cross-link. |
The quantitative test for the self-cleaning capability of the cotton textile was conducted by measuring the absorbance of malachite green. The result (Figure 9) indicated that the efficiency of self-cleaning capability was higher in CAA cross-link (95.59%) as compared with BTCA cross-link (69.96%) under the UV irradiation for 60 min. This finding is also in accordance with the previous result by means qualitative test showing the cotton fiber treated by CAA cross-link acquired a higher degradation capability against malachite green as compared with BTCA cross-link.
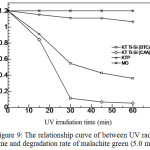 |
Figure 9: The relationship curve of between UV radiation time and degradation rate of malachite green (5.0 mg. L-1). |
Conclusion
In this recent study, a self-cleaning textile cotton fiber coated by TiO2/chitosan/SiO2 nanocluster was successfully developed. The TiO2/chitosan/SiO2 morphologically depicted an anatase structure, with 13.1 mm of crystallite size and a band gap (Eg) = 3.20 eV. This particle acquired the self-cleaning capability against malachite green dye under the UV irradiation (λ= 365 nm; 536 Lux). The increase of self- cleaning efficiency was determined by cross-link functionalization in consistent with the abundance of TiO2/chitosan/SiO2 coating on the cotton fiber. The gradual cross-link functionalization using CAA could highly optimize the self-cleaning efficiency (96.0%) as compared with BTCA (69.96%). The functional optimization of CAA was noticeably correlated with the morphological features revealed by SEM, XRD, and FT-IR analysis.
Acknowledgements
The authors thank the Directorate of Research and Social Services of the Ministry of Research, Technology and Higher Education of Indonesia for funding this research (Competency-based Research Grant Scheme,the fiscal year 2018) and also thank the division of research and social services of Andalas University for facilitating this grant.
Conflict of Interest
There is no conflicts of interest.
References
- Zhang, D.; Ling, C.; Chuanfeng, Z.; Yuyue, Z.; Hong, L. J. Carbohydrate Polymer. 2013, 8, 2088-2094.
CrossRef - Shaban, M.; Semsem, A.; Ahmed, A.K. J. Basic app. Sci. 2016, 5, 277-283.
- Rilda, Y.; Fadhli; Syukri; Admin, A.; Hermansyah, A.; Sheela, C.; Hadi, N. J. Technol. Eng. 2016, 78, 113-120.
- Wijesena, R. N.; Nadeeka, D.T.; Rangana, P.; Nalin, D.S.; Gehan, A. J. Molecular Catalysis A. 2014, 398, 107-114.
CrossRef - Karimi, L.; Mirjalili, M.E.; Yazdanshenas, A.; Nazari. J. Photochemistry and Photobiology. 2010, 86, 1030-1037.
CrossRef - Deyong, W. J. Surface Coating Technol. 2009, 203, 3728-3733.
CrossRef - Pakdel; Esfandiar; Walid, A.; Daoudb; Xungai, W. App. Surface Sci. Australian Future Fibres Research and Innovation Centre, Institute for Frontier Materials, Deakin University, Australia. 2013, 275, 397-402.
- Nazari, A.; Montazer. J. Carbohydrate Polymers. 2011, 83, 1119-1127.
CrossRef - Clara, A.S. J. Sol-Gel Sci. Technol. 2015, 73, 118-126.
CrossRef - Balachandran, K. J. Spectrochim Acta A Mol Biomol Spectrosc. 2014, 128, 468-474.
CrossRef - Meilert, K.T.; Laub, D; Kiwi, J. J. Molecular catalysis. 2005, 237, 101-108.
CrossRef - Aksoy, Sennur, A. Cellulose Chem. Technol. Textile Enginering Department Sileyman Demirel University. 2013, 49, 405-413.
- Zayyim, E.O. J. Material Sci. 2005, 40, 1345-1352.
CrossRef - Li, W.; Xiahong, X., Siyao, C.; Xingping, Z.; Lan, L.; Dajun, C.; Xiaqin, W. J. Carbohydrate Polymers. 2008, 71, 574-58.
CrossRef - Jiang, R.; Zhu, H.; Chenc, J.; Yaoa, B.Y.Q.; Fub, Z.Y.; Zhanga, Y.M.; Xu. App. Surface Chem. 2014, 319, 189-196.
CrossRef - Galkina, O.L. J. Surface Coating Technol. 2014, 253, 171-179.
CrossRef - Jiang, R.; Zhu, H. Y.; Chenc, H.; Yaoa, J.; Fub, B. Y. Q.; Zhanga, Z. Y.; Xu, Y. M. App. Surface Chem. 2014, 319, 189-196.
CrossRef - Rilda,Y.; Reza, S.; Anthoni, A.; Nasril, N.; Achmad, S.; Hadi, N. J. Chin. Chem. Soc. 2017, 64, 1347–1353.
CrossRef - Rilda, Y.; Admin, A.; Edison, M.; Baharuddin, S.; Stefani, K. Asian J. Chem. 2015, 27, 3983-398.
CrossRef - Aziz; Radhiyah, A;. Iis, S. Department of Manifacturing and Material Engineering Faculty Engineering International Islamic University Malaysia. 2009, 48 A, 951-957.
- Junling, L. J. Physic. Chem. C. 2009, 112, 12412-12418.
- Chen, C. C.; Lu, C. S.; Chung, Y. C.; Jan, J. L. J. Hazardous Mat. 2007, 141, 520–528.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









