Some Novel Manganese(III) Mixed Ligand Complexes and its Decolourization Studies
Deepika Jaiswal and Sudha Yadava
Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur, U.P, India.
Corresponding Author E-mail: dr_sudhayadava@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/340625
Article Received on : 17-08-2018
Article Accepted on : 03-11-2018
Article Published : 02 Nov 2018
Some novel mixed ligand complexes of Mn(III) with glycine ligand namely [Mn(gly)2 Cl(en)], [Mn(gly)2 Br(en)], [Mn(gly)2 N3 (en)] and [Mn(gly)2 NCS(en)] have been synthesized starting from Mn(gly)2 Cl, Mn(gly)2 Br, Mn(gly)2N3 and Mn(gly)2 NCS respectively. These newly synthesized complexes have been characterized by UV/Vis, FT-IR and Mass spectrometry. The spectroscopic data suggest distorted octahedral geometry for all these mixed ligand complexes. The λmax values of these complexes for 5T2g → 5Eg transitions are 482 nm for [Mn(gly)2N3(en)], 488 for [Mn(gly)2NCS(en)], 486 for [Mn(gly)2 Br(en)] and 484 for [Mn(gly)2Cl(en)], all these transitions are red shifted in comparison to their parent complexes. The ligand field parameters such as 10 Dq, B and β have also been calculated and suggest covalent metal ligand bonding. One peculiar finding is that the FT-IR spectra shows frequencies for both free and coordinated NH2 group in all complexes indicating that the ethylenediamine ligand present here is non bridging in nature. The mass spectrometry results show molecular ion peaks at m/z 300, 345, 307 and 323 for [Mn(gly)2Cl(en)], [Mn(gly)2Br(en)], [Mn(gly)2N3(en)] and [Mn(gly)2NCS(en)] respectively. The coordination of ethylenediamine to Mn(III) enhances its efficiency towards decolourization methyl red dye.
KEYWORDS:Dye Decolourization; Ethylenediamine; Glycine; Mn(III) Mixed Ligand Complexes
Download this article as:| Copy the following to cite this article: Jaiswal D, Yadava S. Some Novel Manganese(III) Mixed Ligand Complexes and its Decolourization Studies. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Jaiswal D, Yadava S. Some Novel Manganese(III) Mixed Ligand Complexes and its Decolourization Studies. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=51912 |
Introduction
Synthesis, characterization, and applications of Mn(III) complexes had been the focus of global research interest due to their role in the chemical and biological system.1-7 Mn(III) complexes supported on Fe3O4 nanoparticles had been used for selective oxidation of thiols to sulphides.8 Mn(III) porphyrin anchored onto multiwall carbon nanotubes had been used as an efficient and reusable catalyst for heterogeneous reduction of aldehyde and ketones.9 Mn(III)-Schiff base dicyanamide complexes had been used for checking the rhombicity effect in peroxidase studies.10 The application of encapsulated Salen- and Salhd-Mn(III) complexes in an AP- pillared clay for bicarbonate assisted epoxidation of cyclohexane had been demonstrated.11 Mn(III) had been found to play an important role at the redox centers of biological systems, important among those are Mn containing catalase, Mn containing ribonucleotide reductase, and the oxygen evolving center of photosystem II (PS-II).12 Attempts had been made to mimic the active site of these enzymes also.13-15 In most of the cases, Mn(III) complexes have been synthesized using potassium permanganate or Mn(II) and Mn(III) acetate.16-19 However, most of the reported Mn(III)-complexes have octahedral or square pyramidal geometry.15,20-21
The above studies prompted the authors’ group to initiate the studies on the synthesis, characterization and application of Mn(III) complexes. In an earlier, study synthesis and characterization of novel complexes of Mn(III) with macrocyclic porphyrin ligand and ethylenediamine have been reported by this group.22 The depolymerization activity of these complexes towards humic acid as coal model had also been reported earlier.23 Recently Mn(III)-porphyrin complexes with oxidation property have been reported by our group.24 Another work on synthesis and characterization of a novel Mn(III)-(-diketone) complex with catalytic and antifungal activity have also been reported from our laboratory.25 Tris (glycinato) Mn(III) complex has been synthesize for the first time in our laboratory.26 There is a report in the literature27 that coordination of imidazole to Mn(III) in a Mn(III)-porphyrin complex enhances the catalytic activity of the complex towards the oxidation of benzyl alcohol to benzaldehyde in presence of sodium periodate. Considering the above report, the authors have synthesized and characterized ethylenediamine mixed ligand complexes of Mn(III)(gly)2 Cl, Mn(III)(gly)2 Br, Mn(III)(gly)2NCS, and Mn(III)(gly)2N3 and have studied the decolourization potential of these novel complexes towards decolourization of methyl red dye. It has been observed that substitution of ethylenediamine in Mn(gly)2X complex enhances its efficiency as a heterogeneous catalyst towards the decolourization of Methyl Red in presence of H2O2.
Materials and Methods
Glycine was purchased from Mumbai (sigma-aldrich). All the reagents and solvents were of analytical grade and procured from Merck Ltd., Mumbai (India) and used without further purification. Mill-Q water had been used throughout the experiments.
Characterization
The purity of these complexes was checked in our laboratory by TLC method using Silica gel on glass plates. The mobile phase was dichloromethane and methanol 7:3 (v/v). Detection was made by keeping the TLC glass plates in iodine-chamber. The complexes were analyzed for C, H, and N element in Sophisticated Instrumentation Center of Cochin University of Science and Technology, Cochin. The UV-Vis spectra of the complexes were recorded on UV-Vis Spectrophotometer Hitachi (Japan) model U-2900 available in our laboratory. The FT-IR spectra of the complex were recorded on Perkin Elmer FT-IR spectrometer 2000 in KBr disk at the Indian Institute of Technology Kanpur. Magnetic susceptibility measurement data for all these Mn(III) complexes were recorded by the instrument VSM (vibrating sample magnetometer) at IIT Kanpur. DART mass spectra of complexes were recorded on Reservoir, JMS-T 100 LC at the Sophisticated Analytical Instrumentation Facility, Central Drug Research Institute, Lucknow.
Synthesis
Preparation of Ethylenediamine Complexes, [Mn(gly)2X(en)]
All these [Mn(gly)2X(en)] complexes were prepared according to the earlier reported procedure in the literature [26,28] where X= Cl‾, Br‾, N3‾, NCS‾.
For the preparation of azido complex, 0.82 g Mn(gly)2N3 complex was refluxed with 1 mL of ethylenediamine for 18 h. After refluxion the obtained product was cooled for1 h at room temperature (27ºC) resulting in dark black powder of Mn(gly)2 X(en) complex, which was dried under vacuum over P2O5 and washed with mill Q water. The Cl‾, Br‾ and NCS‾ ethylenediamine complexes were prepared analogously.
The reaction scheme for all these synthesized complexes is given below.
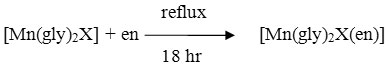
Where gly = glycine, X = Cl, Br, NCS and N3
Catalysis of Methyl Red by the Complexes
The reaction solution (2ml) consisted of 0.24% methyl red, 14.63% H2O2 and 0.5 mg of the complex in milli Q water Since the complexes were insoluble in the reaction medium hence acted as heterogeneous catalysts. The time of 100% decolourization of the dye was recorded with the help of a stopwatch by visual observations. The UV/Vis spectra of the reaction solution before and after the decolourization of dye was recorded.
Results and Discussion
The objective of this communication has been clearly stated in the introduction section. Here ethylenediamine added complexes of Mn(gly)2Cl, Mn(gly)2Br, Mn(gly)2N3 and Mn(gly)2NCS have been synthesized to see if the catalytic properties of these novel mixed ligand complexes towards the decolourization of dye in presence of H2O2 is enhanced by the addition of ethylenediamine.
The purity of these complexes was checked by thin layer chromatography on silica gel glass plates. A Single spot for each of the complex indicates that these complexes are pure. All the physical properties of these complexes are summarized in Table 1. The colour is black in all, it indicates that the colour of the complexes is governed by the presence of Mn(III) ion.
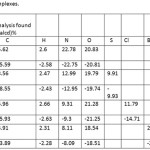 |
Table 1: Physical properties of Mn(III) Complexes. |
The UV/Vis spectra of the complexes were recorded in dimethyl sulphoxide (DMSO). One of the spectrum for Mn(gly)2Cl(en) is shown in Fig.1 while the λmax values for these four complexes are given in Table 2. The λmax and the molar extinction coefficient values for this typical spectrum are 484 nm and 2.142×103 M-1 cm-1 respectively. The molar extinction coefficient value ε484 for this complex is in the range of spin allowed transition 5T2g → 5Eg.29 The λmax values for all these novel mixed ligand complexes are red shifted in comparison to their parent complexes, thus showing the decrease in the crystal field stabilization energies of these complexes. The crystal field stabilization energies of Mn(gly)2Cl(en), Mn(gly)2Br(en), Mn(gly)2N3(en) and Mn(gly)2NCS(en) have been evaluated as 23674 cm-1, 23466 cm-1, 23854 cm-1 and 2333 cm1 respectively whereas the crystal field stabilization energies of Mn(gly)2Cl, Mn(gly)2 Br, Mn(gly)2 N3 and Mn(gly)2NCS are 29,069cm-1, 28409 cm-1, 25201cm-1 and 27173 cm-1respectively.26 In this way the ethylenediamine complexes are relatively less stable than their parent complexes. The ligand field parameters such as 10 Dq, B and β have also been calculated for these ethylendiamine Mn(III) complexes and are summarized in Table 2. The values of Racah interelectronic repulsion parameter (B) are below the free ion value for Mn(III) ion (1140 cm-1)30 it confirms the covalent nature of metal ligand bonds present in all four complexes while the covalency factor β is varying in the range of 0.75±0.88.
![Figure 1: UV/Visible spectra of chlorobis (glycinato) Mn(III) ethylenediamine [Mn(gly)2 Cl (en)] complex.](http://www.orientjchem.org/wp-content/uploads/2018/11/Vol34No6_Som_Dee_fig1-150x150.jpg) |
Figure 1: UV/Visible spectra of chlorobis (glycinato) Mn(III) ethylenediamine [Mn(gly)2 Cl (en)] complex. |
Table 2: λmax values and Ligand Field Parameter of all these mixed ligand complexes.
| Complexes | λmax | 10Dq(Cm1) | B(Cm-1) | β |
| Mn(gly)2N3(en) | 482 | 23,854 | 883 | 0.77 |
| Mn(gly)2NCS(en) | 488 | 23,320 | 863 | 0 .75 |
| Mn(gly)2Br(en) | 486 | 23,496 | 870 | 0.76 |
| Mn(gly)2Cl(en) | 484 | 23,674 | 876 | 0.76 |
![Figure 2: DART Mass spectra of azidobis (glycinato) Mn(III) ethylenediamine [Mn(gly)2N3(en)] complex.](http://www.orientjchem.org/wp-content/uploads/2018/11/Vol34No6_Som_Dee_fig2-150x150.jpg) |
Figure 2: DART Mass spectra of azidobis (glycinato) Mn(III) ethylenediamine [Mn(gly)2N3(en)] complex. |
The magnetic moment values for all these manganese(III) complexes have been found in the range of 4.20- 4.90 BMat room temperature. It confirms the presence of four unpaired electrons and a high-spin d4 system in all the four novel complexes.33,34 On the basis of all the above studies distorted octahedral structure has been proposed for all the novel mixed ligand complexes and it is given as in Fig 4.
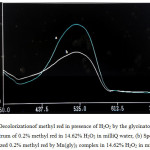 |
Figure 3: Decolorizationof methyl red in presence of H2O2 by the glycinato complex. (a) Spectrum of 0.2% methyl red in 14.62% H2O2 in milli Q water, (b) Spectrum of decolourized 0.2% methyl red by Mn(gly)3 complex in 14.62% H2O2 in milli Q water. |
![Figure 4: Structure for all these synthesized complexes, [Mn (gly)2X(en)] Where X= Cl‾, Br‾, N3‾, NCS‾](http://www.orientjchem.org/wp-content/uploads/2018/11/Vol34No6_Som_Dee_fig4-150x150.jpg) |
Figure 4: Structure for all these synthesized complexes, [Mn (gly)2X(en)] Where X= Cl‾, Br‾, N3‾, NCS‾ |
Now a days oxidation and removal of synthetic dyes from the environment has become one of the important research areas to control environmental pollution. These novel complexes have been tested for their ability to decolourize methyl red in presence of H2O2. The UV spectrum of decolourization reaction of Methyl Red using the initial [Mn(gly)3] is shown in Fig. 3 here Fig 3 (a) is the spectrum of 0.2% methyl red in 14.63% H2O2 in milliQ water and Fig. 3 (b) is the spectrum of the dye decolourized in presence of [Mn(gly)3] complex. In separate experiments, relative efficiencies of [Mn(gly)3], [Mn(gly)2Cl] and [Mn(gly)2Cl(en)] have been tested towards their ability to decolourize Methyl Red dye. The composition of reaction solution and time required for complete decolourization of the dye are given in Table 4.
Table 3: FT-IR spectra of all these complexes.
| Mn(Gly)2 N3en | Mn(Gly)2NCSen | Mn(Gly)2Clen | Mn(Gly)2Bren | Assignment |
| 604 | 599 | 613 | 665 | ν(Mn-O) |
| 487 | 497 | 510 | 486 | ν (Mn-N) |
| 1385 | 1320 | 1320 | 1320 | ν (CH2) |
| 1648 | 1635 | 1625 | 1651 | ν (COO) |
| 2040 | —– | —– | —– | ν (N3) |
| —– | 2090 | —– | —– | ν (NCS) |
| 3400 | 3400 | 3400 | 3368 | ν(NH2)coordinated |
| 3583 | 3583 | 3583 | 3583 | ν(NH2)free |
Table 4: The results of decolourization of methyl red by the complexes.
| S.No. | Complexes | Quantity | Decolourization Time |
| 1. | Mn(gly)3 | 0.5 mg | 10 sec |
| 2. | Mn(gly)2Cl | 0.5mg | 90 sec |
| 3. | Mn(gly)2Clen | 0.5mg | 5 sec |
The order of efficiency of decolourization for methyl red in presence of these complexes can be given as [Mn(III) (gly)2Cl(en)] [Mn(gly)3] ˃ [Mn(III)(gly)2Cl]. The time required for the complete decoulourization of 0.24% Methyl Red in the presence of 14.63% H2O2 in case of Mn(gly)2 Cl(en) complex is only five second while in case of Mn(gly)3 it is 10 second and in case of Mn(gly)2Cl it is 90 second. These complexes have been used for three cycles of catalysis without any apparent change in their these complexes are reusable efficient heterogeneous catalysts for dye decolourization and in this way may be used on the industrial scale for the removal of environmental pollution caused by organic dyes.
Conclusion
Four novel complexes [Mn(III)(gly)2Cl(en)], [Mn(III)(gly)2Br(en)], [Mn(III) (gly)2 SCN(en)], and [Mn(III)(gly)2N3 (en)] have been synthesized and characterized by UV-Visible, FT-IR, Mass spectrophotometry and magnetic susceptibility measurements. Their tentative structure has been proposed to be octahedral. These ethylenediamine mixed ligand complexes of Mn(III) are better reusable heterogeneous catalysts for the decolourization of Methyl Red in presence of H2O2.
Acknowledgments
The authors are thankful to CSIR New Delhi for the financial support through the grant no. 02(0261)/16/EMR II dated 28/04/16. The authors are also thankful to the Head Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur for administrative support. The authors acknowledge the services rendered by the Sophisticated Analytical Instrument Facility (SAIF), Cochin and Central Drug Research Institute (CDRI), Lucknow for recording FT-IR and DART Mass of the samples and IIT Kanpur (ACMS Department) for magnetic susceptibility measurements.
References
- Maxwell, J. G.; Peter, T. Coordination. Chemistry. Reviews., 1991, 108, 115-161.
CrossRef - Jelle, B.; Minze, T. R.; Ronald, H.; Ben, L. F. Inorganic Chimica Acta., 2002, 337, 75-82.
CrossRef - Sumitra, M.; Sanjay, K.; Mandal, S. B.; William, H. A. Chem. Rev., 2004, 104, 3981-4026.
CrossRef - Suzana, C.; Caslav L.; Goran, N.; Jakov S.; Milos. R.; Miladin, G. M. Sensors., 2006, 6, 1708- 1720.
- Donald, J. D.; Eric B. F. Inorg. Chem., 2007, 46, 15, 5967–5978.
CrossRef - Qian, P.; Shi, Z.; Hao, S.; Nian, Li.; Yu-Ping, T; Wei, Li.; Hao T.; Wei- Z.; Min-Zhe S.; Jin, A. D. Current Organic Chemistry., 2013, 17, 2936-2970.
- Chetan, K.; Modi, P.; M. Trivedi. Journal of Coordination Chemistry., 2014, 67, 22, 3678-3688.
CrossRef - Mojtaba, B.; Mohammad, M, H.; Firouz. M. Journal of Coordination Chemistry., 2013. 66, 17, 3025–3036.
- Saeed, R.; Elaheh, B.; Saeed, Z. Journal of Coordination Chemistry, 2016, 638-649.
- Manuel, R. B.; Rocio, C.; Fernandez, G.; M, Isabel.; Ana, M.; G, N.; Gustavo, G. R.; Marcelino, M.; Laura, R. S. Journal of Chemistry., 2017, 1-10.
- Garcia, A. M.; Moreno, V.; Delgado, SX.; Ramirez, AE.; Vargas, LA.; Vicente, MA.; Gil, A. Journal of Molecular Catalysis A- Chemical., 2016, 416, 10-19.
CrossRef - Dismukes, G. C.; Bioinorganic Catalysis, J. Reedijk, (Ed) First edition, Marcel Dekker Inc, New York, 1993, 317.
- Faulkner, K. M.; Stevens, R. D.; Fridovich, I. Arch Biochem Biophys., 1994, 2, 341-346.
CrossRef - Christian, R.; Goldsmith, A. P.; Cole. T. Daniel; P. S. J. Am. Chem. Soc., 2005, 127, 27, 9904–9912.
- Arpan, D. S.; Biswas, M. D.; Bikash, K. S.; Abhishake, M.; Shyamal, K. S.; Mahammad A. RSC Advances., 2015, 5, 23855-23864.
CrossRef - Hossein, A. ; Gus, J. P., Inorg. Chem. 1982, 21, 3903-3907.
CrossRef - Shalaby, M. S.; Abdallah. H., Front. Chem. Sci. Eng. 2013, 7(3) 329–337.
CrossRef - Iffet, S.; Necla, G.; Turgut G., Synth. React. Inorg. Met.-Org. Chem., 2001, 31(7), 1175– 1187.
CrossRef - Altan, Guvenc.; Karabacakoglu, B., Turk J Chem 2000, 24, 101-108.
- Bhagwan, S. G.; Asha, L. D.; Ranjna, D., Transition. Met. Chem. 1988, 13, 351-355.
- Laurence, J. B.; Victor, W. Day.; Inorganic Chemistry, 1977, 16, 1360-1667.
- Sudha, Y.; Shashi. L. B. J. of Coordination Chem., 2011, 64, 3950-3959.
- Shashi, L. B.; Sudha, Y. J. of Coordination Chem., 2012, 65, 3492-3501.
CrossRef - Niharika, A.; Sudha, Y. J. of Coordination Chem., 2018 (accepted).
- Preeti, D.; Sudha. Y. J. of Coordination Chem., 2018. (accepted).
- Deepika, J; Sudha, Y., Inorganica Chimica Acta., (communicated).
- Jelle, B.; Minze T.; Ronald, H.; Ben, L. F. Inorganica Chimica Acta., 2002, 337, 75-82.
CrossRef - Manabendra, N. B.; Mihir, K. C.; Darlando, T. K., Dalton Trans., 1982, 669-670.
- Patel, I. A.; Bharat, T. T., Indian. J. of Chemistry., 1999, 38, 422-433,
- James, E. H.; Ellen, A. K.; and Richard, L. K. Inorganic Chemistry, Principles of Structure and Reactivity, 4th Edn, Pearson Education, Singapore., 2005, 445.
- Stults, B. R.; Robert, S. M.; Victor, W. D. Inorganic Chem., 1975, 14, 4, 722-730.
CrossRef - Brahim, B.; Ali. O.; Djouhra, Aggoum.; Ramiro, R. R.; Yasmina, O.; Emilia, M. Res Chem. Intermed., 2016, 42,(5), 4839–4858.
- Behere, D. V.; Samaresh, M. Inorganic. Chem., 1980, 19, 992-995.
CrossRef - Fleischer, E. B.; Palmer, J. M.; Srivastava, T. S.; Chatterjee, A. J. Am. Chem. Soc., 1971, 93, 3162-3167.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









