Role of Tio2 Pillared Bentonit and Catalyst Nickel for Hydrogenation Glucose to Generate Sorbitol
Minto Supeno, Nurhaida Pasaribu and Rikson Siburian
Department of Chemistry, Universitas Sumatera Utara, Medan-Indonesia.
Corresponding Author E-mail: riksonsiburian2000@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340619
Article Received on : 16-08-2018
Article Accepted on : 01-11-2018
Article Published : 03 Nov 2018
Glucose hydrogenation using water at solvent was happened trough two step. First, water was reduced to hydrogen and oxygen gases by TiO2 pillared bentonite. Second, hydrogen that produced was used to reduce glucose into sorbitol. The reaction was prepared in transpaharan medium for 30 days along under UV from sunlight. The product of reaction was characterized using SEM, XRD, FTIR and DSC.
KEYWORDS:Glucose; Nickel; Pillariedbentonit; Sorbitol; Tio2
Download this article as:| Copy the following to cite this article: Supeno M, Pasaribu N, Siburian R. Role of Tio2 Pillared Bentonit and Catalyst Nickel for Hydrogenation Glucose to Generate Sorbitol. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Supeno M, Pasaribu N, Siburian R. Role of Tio2 Pillared Bentonit and Catalyst Nickel for Hydrogenation Glucose to Generate Sorbitol. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=51942 |
Introduction
Clay mineral is one of Indonesia’s abundant natural resources and has not been used optimally. Clays geologically is a natural mineral from the family of crystalline silicates with a layered structure. Bentonite is a type of clay that is widely available in Indonesia which there are in most areas of Nusa Tenggara, Sulawesi, West Java, Central Java, Yogyakarata, East Java, South Sumatra, Jambi, North Sumatra.1
Bentonite has a strong capability of colloids, when mixed with water it can expand. Principle alter the surface and pore bentonite is to convert the metals contain in the pores become wider. Another method to expand the pores is pillaring, in this case pores bentonite containing Na and K metal intercalated with metals of larger diameter that make the pores expand, then calcined at a temperature of 300-500oC, the metals will forming an oxide layer bonded to produce bentoniteterpilar. In North Sumatra there are two types natural bentonite, Wyoming bentonite and non Wyoming bentonite, and both have the main composition of SiO2/Al2O3 with (4-6: 1) ratio. Bentonite is the common name of a type of clay that can be used to adsorb color, oils, fats and waxes. Pale land is a silicate of a variety of compositions, the main constituent of SiO2 and Al2O3 containing water and chemically bonded. In addition to the above two compounds bentonite also contains CaO, MgO, Fe2O3, Na2O and K2O. Based on the theory of Davis and Masser, that differences in levels of SiO2 and Al2O3 ratio will affect the active power. Soil that has a big ratio of SiO2 and Al2O3 is best soils adsorb. While land with a small ratio of SiO2 and Al2O3 have the small ability to adsorb. Comparison of SiO2 and Al2O3 for a good bentonites 5-6 : 1, which is able to adsorb, and has a large surface area.
Bentonite has a strong capability of colloids, when mixed with water, it can inflate (Wyoming). Bentonite in the dry state beige to green with a specific gravity of 2.4 to 2.8 g/mm3 and a melting point between 1330-1430° C. Natural bentonite generally contain little calcite, carbonate, gypsum and quartz. Surface and pores natural bentonite can be enlarged with the activation of chemical and physical techniques,2 or by using pillaring elements Zr, Ti, Fe, Na, Ca through intercalation technique and calcination at 450°C to produce pillared bentonite called photocatalyst powder.3,4
Semiconductor photocatalyst powder has been widely studied, has been found that the activity of the photocatalyst is getting better with decreasing particle size causes increased surface area. A decrease in particle size between 5-10 nm causes changes in energy band structure of the semiconductor becomes known as a side effect Quantum. Further research has been done to produce photochemical of various sizes and shapes, particles of semiconductor Cholocogenide such as CdS, ZnS, CdSe, GeSe, ZnSe and oxide semiconductors of the type ZnO, Fe2O3, TiO2 has been widely used to photocatalysts for producing hydrogen from water.5
Principle alter the surface and pore bentonite was dissolving the metals contained in the pores of bentonite with an acid and metal is already late because the pores become wider. Another method to expand the pores by means pillaring, in this case pores bentonite containing Na and K metal intercalated with metal cations diameter is larger so that the pores inflates, then calcined at a temperature of 300-500°C.6,7 The metals will form oxides bonded to inter-layers, resulting pillared bentonite.4 Through this technique would be great porosity bentonite, metal oxides as pillaring agent can be used for the catalyst.
In this research, intercalation bentonite pores using TiO2 and calcination temperatures of 300-500°C to produce TiO2 – pillared bentonite. Its isolator part i.e oxides can be etched to remove oxides by using a mixture of HF / H2O / NH4F or HF / HNO3 / H2O or by using CF4 / H2 which produces silicon layer that is free from oxide and silicon is then etched with a solution of HF / HNO3 / CH3COOH / I2 so that the silicon will be dissolved. The amount of surface area that is produced depends on the time used for etching. If the time spent too long SiO2 or Si late at all and thus it is not expected that the time used for etching needs to be controlled.8,9
If the etching technique is achieved then the surface and pores become larger pillared bentonite which allegedly producemacropores pillared bentonite. Pemilaran by using TiO2 and etching silicate bentonite can change the physical and chemical properties, increase the basal spasing (D001), specific surface area, total volume, surface acidity and decrease the average pore spokes.
TiO2 pillared bentonite can be used as catalysts in the manufacture of hydrogen gas and oxygen from the water, in this study the researchers are interested in examining the provision of this pillared bentonite as catalyst.
Natural bentonite has 60 % of its silicon content, to provide this material as a catalyst it is necessary to increase the surface area and pore volume by way of intercalation with TiO2 and be-TiO2 pillared bentonite. Titania metal oxide is a material that is sensitive to light and either be a photochemical catalyst. If TiO2 pillared bentonite do etching with chemicals then etched pillared bentonite can be co-catalyst. So that needs to be studied preparing a catalyst sensitive to sunlight than natural bentonite and bentonite pillared whether TiO2 that has been etched can be as a co-catalyst manufacture hydrogen and oxygen gases from water.
Aldehyde group of glucose may experience a reduction in the presence of hydrogen and a catalyst to form a metal hydride into polyalcohols called alditol. Product of the reduction of d-glucose called D-sorbitol or sorbitol.10 Past research has made the hydrogenation of glucose to sorbitol with different catalysts11, where it was made from the hydrogenation of glucose 50% at a temperature of 353 K, a pressure of 4 MPa using a Ni catalyst. Clauss, 2006 using a catalyst Ni / SO2 with glucose 20 % at temperature of 393 K with pressure 120 bar. It also hydrogenated glucose with Nickel catalyst at temperature 95-105o C and pressure 60 bar. From the past research’s result, that generated 0.5963 g sorbitol (g sorbitol / g g sorbitol early).12
Based on the description above, the researchers are interested to hydrogenate glucose to sorbitol without pressure and high temperature. With the TiO2 pillared bentonite as producing hydrogen gas from water using UV light from solar energy.
Materials and Methods
The Materials Used in This Study Include
The bentonite clay was taken from the District Padang Tualang, Langkat, North Sumatera, which have passed the 100 mesh sieve.
Chemical reagents with quality p.a was made by E.Merck as follows: TiCl4, concentrated HCl, H2SO4, AgNO3, BaCl2, NaCl, ethanol, HF, NH4F, CH3COOH, I2.
Distilled Water and Demineralized Water
Bentonite clay with a composition of 61.0 2 % SiO2; 15.21 % Al2O3; 4.89 % Fe2O3; 0.62 % TiO2; 2.08 % CaO; 1.94 % MgO; 0.46 % K2O; 3.45 % Na2O; 10.3 1% incandescent lost. Based on this, the composition of bentonite District of Padang Tualang, Langkat, types of Na-bentonite. Bentonite is sieved to 100 mesh sieve passes are then washed with distilled water several times and filtered by vacuum filtration and dried in an oven at 100° C for 5 hours. After that bentonite clay is dried and crushed into powder and sieved using a 100 mesh sieve.
Provision of Na-Bentonite
One hundred grams of bentonite clay (3.3) was dispersed into the 1.5 l NaCl 1 M and submergeded for 1 week in which every two days NaCl solution was replaced with a new one. At every replacement of NaCl stirring for 24 hours by heating to 60-70° C for 4 hours, then after its precipitate filtered and then washed with demineralized water until free of chloride ions, evidenced by negative silver nitrate test. Filtering was done using vacuum filters and bentonite obtained dried in an oven of 100°C until dry, then crushed and sieved using a 100 mesh sieve.
Furthermore, bentonite is saturated using 6 M NaCl while stirring for 24 hours, then filtered by vacuum filtration and washed with distilled water until free of chloride ions with negative AgNO3 test. Then dried in an oven at 100°C. After dried crushed into powder and then sieved using a 100 mesh sieve. Results saturation of bentonite clay is called Na-bentonite.
Activation of Na-Bentonite with Acid
Each 35 grams of Na-bentonite dispersed into 150 ml solution of sulfuric acid 0,5; 1; 1.5; and 2.0 M while stirring with a magnetic stirrer for 6 hours. Then allowed to stand for 24 hours and then filtered by vacuum filtration and washed with hot distilled water until free of sulfate ions. This is indicated by a negative test against BaCl2. Activated acid Na-bentonite is then dried in an oven at 100°C. After dried crushed into powder sieved using a 100 mesh sieve size. This product was called the Na-bentonite, the product was tested by X-ray diffraction.
Intercalation and Pillarization Na-Bentonite
Weighting each 30 grams of Na-bentonite clay and dispersed into 1.5 l of deionized water (aquabidest) and stirred with a magnetic stirrer for 6 hours. Then into each Na-bentonite poured piecemeal solution of 0.82 M TiCl4 while stirring with a magnetic stirrer for 10 hours. Results of intercalation separated by vacuum filtration and then washed several times with deionized water until free of chloride ions. Washing stopped if the filtrate was tested with silver nitrate does not form a white precipitate. Bentonite clay that has been intercalated with TiCl4 dried in an oven at 100°C. Once dried crushed into powder and sieved with a 100 mesh sieve then calcined at 350°C. This product is called TiO2-bentonite.6,7
Etching TiO2 Pillared Bentonite
TiO2 pillared bentonite was calcined at a temperature of 400° C, taken 20 g, and then put in a plastic container. Etcher solution is then added (a mixture of: 3ml HF (p) + 5ml HNO3 (p) + 3ml CH3COOH (glacial) / I2 of 0.3 g/250 ml H2O). Then stirred using a plastic stirrer for 10 minutes, then the precipitate is separated from the solution by decantation using a plastic pipette. The precipitate was dispersed in aqua bidestilate then neutralized pH, decanted using a plastic pipette. Etching products are divided into 3 parts, each furnaced at 400, 450, 500°C for 1 hour. Then the lower heated products were analyzed by SEM.
The results of SEM images indicate that the product is heated at a temperature of 450° C has the most extensive surface area and will be used to test the catalyst/co-catalyst in water.
Making Hydrogen and Oxygen Gas from Water Using the catalyst/co-catalyst TiO2 pillared Bentonite by UV irradiation
Bentonite was weighed as much as 4 g, then put in a pumpkin that has been filled in 10 ml of distilled water and stirred for 10-15 minutes then measured the pH. Pumpkin is connected with a thermometer and three branch pipes are connected to a manometer. Subsequently irradiated with ultraviolet as irradiation was carried out for 1-5 days and observed no changes in the manometer. Manometer result of changes in total gas pressure can be calculated total gas (%).
Sorbitol Hydrogenation
We inserted into a transparent bottle containing 2.5 glucose, 0.1 g of nickel powder, 1 g of TiO2 pillared bentonite and 20 ml of distilled water and then sealed. Stirred with a magnetic stirrer and placed under a UV sun rays for 30 days. Then filtered, pH measured and tested with Tollens reagent and Fehling reagents. After that, the filtrate evaporated and sucked up a vacuum to form crystals. Tested melting point and were characterized by FTIR.
Results
Bentonite of Padang Tualang district, Langkat composition show in Table 1.
Table 1: Composition of Bentonit of Padang Tulang District.
| SiO2 | 61,02 % | MgO | 1,94 % |
| Al2O3 | 15,21 % | K2O | 0,46 % |
| Fe2O3 | 4,89 % | Na2O | 3,45 % |
| TiO2 | 0,62 % | missing incandescent |
10,31 % |
| CaO | 2,08 % | Water content |
7,07 % |
Based on the analysis of the composition of bentonite Kabupatan langkat then bentonite above include Na-bentonite type or swelling, bentonite was dried in an oven at 100° C and crushed and sieved to 100 mesh sieve. Bentonite was soaked in 1 M NaCl for 1 week, so going enrichment of Na-bentonite after forming sodium bentonite then put into a 100°C oven until dry and after dry sieved to 100 mesh sieve. The last step enrichment sodium bentonite made by dispersing the Na-bentonite 6 M NaCl solution or saturated NaCl for 24 hours, then washed and dried 100°C, this material is called Na-bentonite.
Na-bentonite subsequently dispersed into several solution of sulfuric acid 0,5; 1; 1.5; 2 M stirred with a magnetic stirrer, activation carried out for 24 hours, filtered by vacuum filtration and then dried in an oven. Activation is aimed to increase the distance between Na-bentonite layer so that it becomes larger.
Once the distance between the layer of Na-bentonite new enlarged carried intercalation and pillarization where activated Na-bentonite dispersed 0.82 M TiCl4 complex solution while stirring with a magnetic stirrer for 18 hours. The results of this intercalation separated by a vacuum pump, intekalasion purpose to enter into the complex Ti distances between the bentonite layer, then calcinated at 350°C to form a more solid oxide pillars.
Analysis was done by X-ray diffraction, using Cu powder radiated by Ka, each 2 grams of TiO2 pillared bentonite and activated clay filled into a sample and then made diffractogram with l = 1.5425 Å.
Based on the results of measurement of basal spacing (D001) there was an increase in basal spacing on the TiO2-pillared bentonite using acid activation of 0.5 and 1.5 M while those using TiO2 pillared bentonite activation damaged. It can be seen from the X-ray diffraction data. The increase in basal spacing will be followed by an increase in surface area, increased porosity, and total volume.
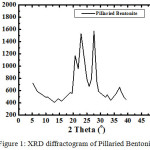 |
Figure 1: XRD diffractogram of Pillaried Bentonite. |
Based on Figure 1, pillaried bentonite was characterized by peaks at 2-theta ie: 5.92; 6.36; 18.84; 29.28 with basal spacing d (A) respectively: 14.91; 13.88; 4.70; 3.04 and other peaks are kaolinite, quartz, mica means bentonite has not been enriched so there are still impurities.
From this diffractogram (Figure 1) can be given information about changes in the angle theta 6 changes the distance between layers of Na-bentonite into a pillared bentonite-TiO2 for observation or pillared bentonite changes in the angle theta 0-5. From Figure 1 and 2 have been changes in peak intensity and changing the distance between layers D001.From the X-ray diffraction data above (Figure 1 and 2) can be determined the distance between layers, as well as identification in identifying the types of clay mineral, to calculate the distance between layers (d) mineral bentonite can be used formula Bragg:
nλ= 2 d Sin θ
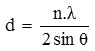
where, d = the distance between the fields of atomic crystals.
λ = wavelength (1 Å = 10-10 m)
θ = angle of diffraction
n = order of diffraction
(a) The distance between layers (d) for the Na-bentonite
n = 1
λ = wavelength (1 Å = 10-10 m)
2 θ = 5.920; θ = 2.960
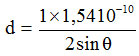
d = 14.917 Å
(b) TiO2 pillared Bentonite using 1.5 M sulfuric acid can be calculated as follows:
n = 1
λ = 1.54 x 10-10 m
2 θ = 5.920; θ = 2.960
d = 16.9807 Å
Further changes in the distance between layers (Δd) are:
(Δd) = d (b) – d (a)
= 16.980 to 14.916
= 2.063 Å
Based on X-ray diffraction analysis then by intercalation and pillarization add, increase the porosity of the basal spacing = 2.06 Å.
Based on the data of three X-RD, a concentration of 1.5 M sulfuric acid good for intercalation in pillarization produce physical changes in basal spacing, surface area and total pore volume increases.
Furthermore TiO2 pillared bentonite activated in best H2SO4 etched using a mixture (28 ml HF + 170 ml H2O + 113 g NH4F) for 2-10 minutes for etching the oxide on silica and make a lot of holes (H+) on silica, then etched using solution (1 ml 5 ml HF + HNO3 + 2 ml CH3COOH + 0.3 g I2 / 250ml H2O) for 5-10 minutes for etching silicon further heated to 400, 450, and 500°C for 1 hour. With such technique will produce pillared bentonite macropore and reproduce (H+).
Based on this data (Table 1) then etching increases the surface area of the surface area of Na-bentonite 89.0563 m2 / g increased to 92.0123 m2 / g so that the average increase surface area of 2.956 m2 / g, this result has been satisfactory. These results were further tested using analysis of surface area (BET), the result is as follows:
Table 1: Surface Area TiO2 pillared Bentonite which has been etched on the Different Temperatures.
| Temperature (°C) | Surface Area (m2.g-1) | Totat Pores Volume (cc.g-1) |
| 400 | 902,387 | 0,0446 |
| 450 | 920,123 | 0,0444 |
| 500 | 911,255 | 0,0444 |
Furthermore TiO2 pillared bentonite photographed SEM showed that the surface be great.
 |
Figure 2: SEM image of etched TiO2 pillared bentonite and heated 450°C. |
Figure 2 shows the number of holes on the surface of the silicate almost entirely on TiO2 pillared bentonite that has been etched. This surface could imply that the TiO2 pillared bentonite has a lot of holes etched then occurs in the silicate external and internal possibilities.
For sorbitol and glucoee, we characterized by using FTIR and DSC (Figure 3 and 4)
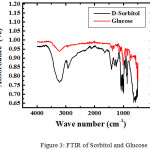 |
Figure 3: FTIR of Sorbitol and Glucose. |
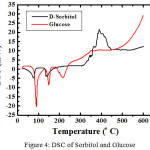 |
Figure 4: DSC of Sorbitol and Glucose. |
Discussion
Preparation of Na-Bentonite
Bentonite samples from the District Padang Tualang, Langkat that have not done enrichment bentonite, made into Na-bentonite produce basal spacing D001 is 14.917 Å, whereas in theory Na-bentonite its basal spacing is 9.8 Å. This means Na-bentonite absorbs water from the humidity so that the measurement time X-ray diffraction to be great. From the X-ray diffraction data (Figure 1) clearly show Na-bentonite which still contains koilinit, quartz and mica. Na-bentonite can be observed a peak at an angle of 0-5 theta, at the height of this is the identity of the Na-bentonite.
Intercalation and Pillarization
Na-bentonite subsequently soaked with sulfuric acid of 0.5-2 M and intercalated using Ti2+ then pillared at 350°C. The calcination is useful to form the pillars of oxide on bentonite. Thus forming a TiO2 pillared bentonite. For the identification of pillared bentonite have seen from the X-ray diffraction data at an angle 0-5 theta, which turned into a basal spacing is 16.9807 Å.
It means, the manufacture of the pillared bentonite has been successful in increasing the basal spacing, surface area and pore volume. The study of literature obtained basal spacing of 28.3 Å. This could occur because of the purity of the bentonite is used, meaning that bentonite material is different then produced basal spacing on different pillars.
Etching TiO2 Pillared Bentonite
TiO2 pillared bentonite subsequently etched using a chemical etcher to reproduce hole (h+). Hole in silicate form marked changes in surface area and pore volume of the original. Also, based on the SEM image surface becomes more rugged than ever before.
As a result of ultraviolet radiation λ= 180 nm, the hydrogen bonding of water will be released and oxygen from water interacting with metal oxides TiO2 and hydrogen from the water molecule will interact with silica. These interactions can lower the activation energy of water molecules. Ultraviolet light enter the pores of bentonite by SiO2 ultraviolet light is converted to shortwave resulting hydrogen and oxygen molecules to break up.
Testing of gas generated a total of 78.5% using pillared bentonite etched, while those using TiO2-pillared bentonite gas generated as much as 60.4%.
Sorbitol FTIR and DSC Spectrum
FTIR spectrum of the product (KBr) showed an absorption band at 3391.2 cm-1, 2935 cm-1, 1646.02 cm-1, 1047.39 cm-1. Absorption at 3391.2 cm -1 region indicates streching stretching vibration absorption OH OH group that stretched from primary and secondary alcohols. Which supported an absorption band 1418.07 cm-1 as buckling OH. Absorption at 2915.7 cm-1 region showed the presence of CH and CH2. While the C-O in alcohol produced ribbons region in 1416 and 1047.39 cm-1. Base on DSC, sorbitol has melting point = 61oC and glucose = 81oC. It means we succeed to synthesize sorbitol base on glucose as a raw material.
Conclusion
In this research, we concluded that hydrogen gas produced by TiO2 bentonite in aqueous solvent can be used for the hydrogenation of glucose to sorbitol. Nickel catalysts and irradiation with UV sunlight for 30 days and produced sorbitol as much as 66.8%
Acknowledgement
We would like to thankful for DRPM-Dikti, Ministry of Research, Technology and Higher Education, Republic of Indonesia who supported the funding for this research.
References
- Al-Qunaibit, M.H.; Mekhemer, W.K.; .The Adsorption of Cu (II) Ion on Bentonite – a Kinetic Study. J. 2004, 1-6.
- Burch, R.; .Pillared Clay. 1997, 283 – 297.
- Barrer, R.M,; .Zeolites and Clay Minerals as Sorbent and Molecular Sieves.2002.
- Bradley, S.M.; Kydd, R.A.; Yamdagni R.; Fyfe, C.A.; .Expanded Clays and Other Microporous Materials: Synthesis of Microporous Materials. 1992, Vol. 2.
- Darby, D.; .Titanium Dioxide Pigment, in Modern Inorganic Chemical Industry. 1997, 31.
- Bask, .Introduction to Colloid Chemistry Interscience. 1992, 15.
- Kharitonova, G.V,; Shein, E.V.; Vityazev, V.G.; Lapekina, C.I,; .Water Vapour Adsorption by Soil Aggregate Fractions. 2004, Vol. 19. 47 – 52.
- Klinowski, J.; .Activation of Alumina-Silica. 1982, 525.
- Ishisaki, K.; Komarmeni, S.;Nanko, M.; .Porous Material Process Technology and Application.,1998, 6–11.
- Darby, D.;.Titanium Dioxide Pigment, in Modern Inorganic Chemical Industry. 1997, No. 31.
- Clauss, P,;. .Ni/SO2 Catalyst Prepared With Ethylendiamine Nickel Precursor : Influence of Pretreatment on The Catalytic Properties in Glucose Hydrogenation. 2006, 45-53.
- Cool, P.; Vansant, E.F.; .Pillared Clays: Preparation, Characterization, and Application. 2002.

This work is licensed under a Creative Commons Attribution 4.0 International License.









