Recent Trifluoromethylation Reactions. A Mini Review Paper
Maher Khalid and Shireen Mohammed
and Shireen Mohammed
Department of Chemistry, Faculty of Science, University of Zakho, Kir-Iraq.
Corresponding Author E-mail: maher-333@hotmail.de
DOI : http://dx.doi.org/10.13005/ojc/340603
Article Received on : 06-11-2018
Article Accepted on : 12-12-2018
Article Published : 14 Dec 2018
This review highlights recent improvement in trifluoromethylene functionalization processes with CF3 reagents, includes: Togni’s, Umemoto’s, CF3SO2Cl, CF3I, Yagupol’skii-Umemoto, TMSCF3, Langlois (CF3SO2Na) and clarifies the several designing to result the corresponding trifluoromethylated products. In this article, the selective trifluoromethylation reactions with Togni’s reagents and their analogs are detailed, which work transition metals or photoactivated Ru or Ir catalysts as single electron giver to yield CF3 radical intermediate species. This issue is to introduce a draft of diverse reports, presenting the modern reaction collect and mechanics produced during the past five years. This task is demanded by the key protocol connected with the trifluoromethylation reactions, designing to sustain researchers a straight forward understanding of such reactions and to award information for further implements.
KEYWORDS:Diastereoselectivity; Photocatalyst Process; Togni’s Reagent; Trifluoromethylation; Umemoto’s Reagent; Langlois’ Reagent
Download this article as:| Copy the following to cite this article: Khalid M, Mohammed S. Recent Trifluoromethylation Reactions. A Mini Review Paper. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Khalid M, Mohammed S. Recent Trifluoromethylation Reactions. A Mini Review Paper. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53916 |
Introduction
The first of fluorine chemistry began with the synthesis and an actual separation of elemental fluorine (F2) by Henri Moissan in 1886.1 Compounds including fluorinated groups are encounter quite interest in pharmaceuticals, materials, and agrochemicals,2 due to the remarkable advantages of it such as great electronegativity impact (4.0 in Pauling scale), steric hindrance, solubility, lipophilic feature, metabolic duration, and bioactivity.3,4 Within fluorine-inclusive functional groups, trifluoromethyl moiety (CF3) is one of the most common and electron-withdrawing group, which result in unusual effect on the pKa value of nearer functional groups, for example, amines, carboxylic acids, and alcohols.5 The growth of actual and efficient of trifluoromethylation (CF3) group into organic framework is highly strenuous research field.6 Moreover, the building block protocol and stereo-planned trifluoromethylation reactions have active method of installing fluorine onto organic compounds framework and the procedure widely used widely used these last years.7 In this mini-review we detail some recent developments of trifluoromethylation of alkenes synthesis and discuss select strategy examples of this process.
In 2014, Yu and co-workers8 disclosed a flexible protocol for installing of CF3 group on the isoquinoline backbone utilized through the photoredox process (Scheme 1). Under the optimal conditions, various of vinyl isocyanide substrates 1 inserted with Umemoto’s reagent 2 as model substrate for the generation of CF3, followed by irradiation step, using white LED (13W), photocatalyst Ir(ppy)2(dtbbpy) PF6 and Na2HPO4 in MeOH at room temperature. The reaction demonstrated its efficiency through the cyclization process, forming the corresponding 1-trifluoromethylisoquinoline products 3 with higher yields. The authors suggested two possible mechanism for the generation of CF3● radical from Umemoto’s reagent supported by visible light photoredox catalyst cycle. The CF3● radical intermediate is trapped by vinyl isocyanide substrate to form imidoyl radical, which can undergo intramolecular cyclization to produce aryl radical. Subsequently, the aryl radical is then oxidized by Ir(IV) catalyst to aryl cation and regenerated Ir(III), followed by deprotonation by base and yield the desired product.
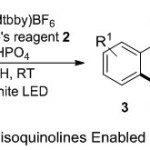 |
Scheme 1 |
While, a flexible access of trifluoromethylation of electron-rich non-terminal styrenes was described by Guo and co-workers (Scheme 2).9 This methodology disclosed the efficient role of Togni’s reagent 5 as a catalyst in the synthesis route of α-trifluoromethyl ketone derivatives 6, under Fluorescent light photoredox conditions. Furthermore, mechanistic experiments suggested two mechanism paths, involving radical processes. In the first path, the Ru(II) catalyst undergoes metal-to-ligand transfer when subjected to light, giving the photoexcited state Ru*(II).on the other hand, Togni’s reagent plays as an oxidative quencher, transform Ru*(II) into Ru(III), and generates a CF3 radical. While the latter radical adds to alkene 4, yielding the final product after abstraction of an oxygen atom from DMSO. Whilst in the second path, Ru(III) catalyst oxidizes the electron-rich alkene substrate into a radical cation intermediate, which undergoes cycloaddition reactions under photocatalyst operation. This intermediate undergoes further stabilization by DMSO, leading finally to combine with CF3 radical and producing cation intermediate then the expected product.
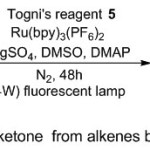 |
Scheme 2 |
Independently, Georg group10 have used trimethyl (trifluoromethyl) silane reagent in the synthesis of medicinally remarkable 3-trifluoromethylpiperidine derivatives 9 (Scheme 3). Under transition Metal-Free reaction condition, theprotocol here relied on using 2,3-dihydropyridin-4(1H)- ones 7 with TMSCF3 8, (PIDA), KF, and promoted by MeCN solvent at room temperature. The advantage of such reaction that can proceed smoothly with cyclic enaminone substrates, including both electron-withdrawing and -donating groups, which proposed a helpful level to yield the expected products with good yields. A sensible mechanistic for such radical reaction proceeds firstly through formation of hypervalent iodine (III) from the ligand exchange between PIDA and TMSCF3 in the presence of KF. Subsequently, the generation of CF3● radical and reaction it with enaminone, produce the cationic intermediate via (SET) process with iodine radical. Finally, the deprotonation by acetate anion yields the desired product.
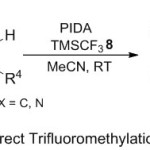 |
Scheme 3 |
While Zhang11 published recent review, included novel and useful methods for selective introducing of CF3 group into various organic compounds framework, by employing trifluoromethylation reactions, with Langlois’ reagent 10 (Scheme 4). Generally, this paper described the efficient role of sodium trifluoromethanesulfinate (CF3SO2Na) as a source generation of CF3● electrophile radical intermediate species in the four main methods of trifluoromethylation reactions, such as: alkenes 11, vinyl- or arylboronic acids 12, 13 and potassium organotrifluoroborates, (arenes, heterocycles) 14 and finally in the cyclization processes 15.
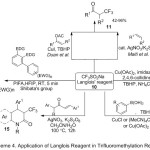 |
Scheme 4 |
Recently, Zhang an co-workers12 detailed the efficient role of BiOBr Nano sheets as photocatalyst in cascade transformation of activated alkenes 16 (Scheme 5). The reaction is value by its wide substrate range, one pot, and simple protocol under mild conditions for the synthesis of α-aryl-β-trifluoromethyl amide compounds in medium to good yields. To set the postulate, N-phenyl-N-tosylmethacrylamide substrate and CF3SO2Cl reagent (CF3 source) 17 are selected as samples in incorporation with BiOBr nanosheets, K2HPO4 in DMAC under photoredox conditions. Generally, this reaction mechanism suggested to proceed smoothly via sequential trifluoromethylation/aryl migration/desulfonylation and N–H bond formation styles. This strategy enables a practical access to a series of α-aryl-β-trifluoromethyl amides 18 bearing a quaternary stereocenter in moderate to good yields.
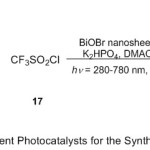 |
Scheme 5 |
Furthermore, Vincent and co-workers13 developed the trifluoromethylation of alkenes reactions via using copper (II) as a pre-catalyst under photocatalyst process (Scheme 6). The protocol here, depended on the treatment range of alkenes 19 with Togni’s reagent as the CF3 source, and photoreducible Cu(II) complex. The reaction mixture managed at ambient temperature in methanol deuterium and displaying to sunlight/ambient light. A sensible mechanistic for such reaction proceeds through generation of CF3 radical intermediate and Cu(II)-carboxylate species from the direct reduction of Togni’s reagent 5 by the copper catalyst, followed by rapid reaction with alkene and producing alkyl copper(III) intermediate. Subsequently, the latter undergoes β-hydride elimination to afford the E-isomer of the allylic trifluoromethylated alkene product 20.
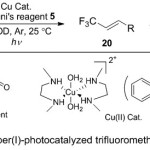 |
Scheme 6 |
While, Cho and co-workers 14 have been reported a simple route towered synthesis of β-trifluoromethyl ketones via alternate radical fashions for the trifluoromethylation and isomerization of propargylic alcohols 22 reactions (Scheme 7). These two reactions allow new access to a variety of aromatic β-CF3 ketones 24 in one-pot. In this case, mixture of readily available propargylic alcohols and trifluoroiodomethane, demonstrate their efficiency through the trifluoromethylation with CF3I 23 and isomerization process in presence of Ru(bpy)3Cl2 as the photocatalyst and DBU under blue light application, forming the corresponding products in higher yields.
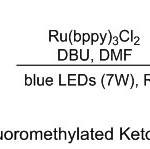 |
Scheme 7 |
Moreover, Liu group15 reported the effective usage of the transformation of readily available of a-(1-hydroxycycloalkyl)-substituted styrene substrates 25 to the relating cycloalkanones 27, containing stereogenic carbon centers (Scheme 8). Remarkably, the yielding of this reaction proceeds through radical and polar mechanisms, firstly by the trifluoromethylation the double bond of styrene in presence of Umemoto’s reagent 26 and [Ru(bpy)3] (PF6)2, under radical photoredox-catalyzed semipinacol-type rearrangement16 process, and secondly by ring expansion of the cyclo-intermediate species via ionic reaction conditions in the same pot. The authors suggested that the addition of trimethylsilyl trifluoromethanesulfonate (TMSOTf) (1.2 equiv) is to protect the hydroxyl group and to decrease the nucleophilicity of it.
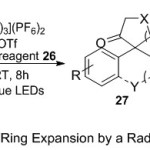 |
Scheme 8 |
In parallel, Akita group17 focused on trifluoromethyl triflation and trifluoromethyl -tosylation of alkyne derivatives, utilized by photoredox catalysis style (Scheme 9). The key incorporation between the 1-phenyl-1-propyne an unsymmetric internal alkyne substrate 27, catalyst [Ir(ppy)2(dtbbpy)] (PF6), and Yagupol’skii-Umemoto reagent 29, encouraging one-pot stereo controlled synthesis of the corresponding tetra-substituted CF3-substituted alkene products 30. Furthermore, the stereo-control of the trifluoromethyl alkenes products bearing triflate (OTf) group were subjected in situ to the Pd-catalyzed coupling reactions. It resulted that the stereochemistry was nearly keep over the Pd-catalyzed reactions under mild condition.
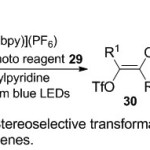 |
Scheme 9Click here to View scheme |
Recently, photoredox process has been employed for the synthesis of CF3– and CF2H-spiroethers from aryl-fused cycloalkenylalkanols. In this context, Akita group18 reported the first example of an oncoming to anti-fluoromethylatedspiroethers 32 with higher diastereo selectivity (Scheme 10). The mild reaction conditions with presence of Umemoto’s reagent (CF3 source) 2, Ru(bpy)3](PF6)2 as catalyst, and 2,6-lutidine in CH2Cl2, the corresponding product derivatives were achieved in excellent yields up to 99%. The authors suggested that the mechanism starts with generation of the fluoromethyl radical intermediate species(●CF2X) via single-electron transfer process between the photo excited of Ru (II) to Umemoto reagent through photocatalytic, followed by radical addition reaction to cycloalkenylalkanol substrate 31 to produce subsequently, two radical intermediates. The latter is then undergoes intramolecular nucleophilic reaction of alcohol from the opposite side, furnishing the expected anti-fluoromethylatedspiroether products.
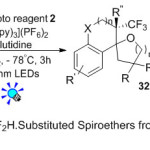 |
Scheme 10 |
Later, a variety of chiral azaheterocyclecores 35 with CF3 group and bearing α-quaternary stereogenic center have been prepared via radical asymmetric amino-trifluoromethylation of alkenes 33 reactions by Liu group (Scheme 11).19 Here, the dual-catalytic system plays two efficient roles, firstly by introducing of a Cu(I)/chiral phosphoric acid and secondly by following up of both nucleophilic and directing properties of two acidic N-H bond of urea. The scope and limitation of this method allowed a positive conversion into other novel compounds in o organic synthetic fields.
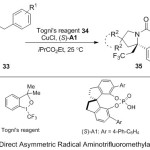 |
Scheme 11 |
While Li and co-workers20 applied Langlois’ reagent for hydrotrifluoromethylation of unactivated alkenes 36 (Scheme 12). The protocol here, relied on using Mn(OAc)3· 2H2O as the oxidant in the presence of readily available sodium trifluoromethanesulfinate (Langlois’ reagent) 10. Generally, the reaction mechanism proceeded smoothly through free radical pathway. The Generation of CF3● radical in situ from the reaction between Mn(OAc)3· 2H2O and CF3SO2Na. Subsequently, the Markovnikov addition of CF3● radical to the double bond of alkene, followed by hydrogen abstraction, produced the desired hydrotrifluoromethylation product. It is noteworthy that variety of functional groups in alkene including alcohol, amide, ether, and ester, played a broad amplitude toward the reaction conditions and affording the expected products 37 up to 78% yields.
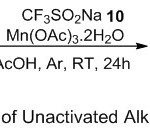 |
Scheme 12 |
Later, Mac Millan group 21 described a novel protocol for transformation of carboxylic acids 38 to trifluoromethyl groups 39, through the incorporation with Togni’s reagent under photoredox copper catalysis (Scheme 13). The reaction forward in significant with, alcohols, olefins, heterocycles, and strained ring system, producing the corresponding products in higher yields. Here, the pathway of decarboxylative trifluoromethylation mechanism starts with generation the oxidizing excited state* Ir (III) from the photoexcitation of the Ir (III) photocatalyst with visible light. In mean time, the reaction of carboxylicacid with base, produce the carboxylate ion, which can then ligate Cu(II) catalyst and delivered Cu(III) carboxylate via single-electron transfer process. Subsequently, the carboxylate ion undergoes dissociation then elimination of CO2 to generate the alkyl radical. At this point, the latter combines with Togni’s reagent and yield the corresponding product.
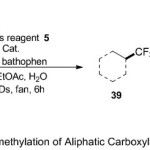 |
Scheme 13 |
Furthermore, Boutureira and co-workers22 performed insertion of CF3 units into a predefined center of electron-rich alkenes 40, using fluoroform-derived “ligandless” CuCF3 (Scheme 14). Here, the reaction, allowed a good incorporation of CF3 group, that based on the selective reaction of iodine at both carbon positions (electron-deficient and electron-rich) of (benzo-fused heterocycle, nucleo base, and glycal) substrates, followed by a novel reaction with “ligandless” CuCF3 via specific cross-couplings with C(sp2)-I bonds.
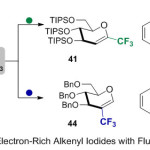 |
Scheme 14 |
While, Xu and co-workers23 described a mild and novel procedure for the azidotrifluoromethylation reaction of N-heterocycles and terpenes, promoted by the iron catalyst and TMSN3 reagent (azido-group) 48 (Scheme 15). The reaction is value by its wide olefin 47, N-heterocycle and terpene 51 substrates range, simple protocol under mild conditions for the synthesis of vicinal trifluoromethyl primary-amine compounds, which are difficult to prepare them with the present approaches. The suggested mechanism proceeds through formation of iron-azide-derived catalyst via activation of Fe(OAc)2-ligand complex directly by TMSN3, followed by generation of CF3●radical through irreversible reduction with Togni’s reagent under Single Electron Transfer (SET) process.The irreversible radical addition of CF3● olefin, produce carbo-radical intermediate species, which can be quickly, deposed by a high equivalent iron-azide moiety through azide-ligand switch, yielding the expected products 49, 50 and 51.
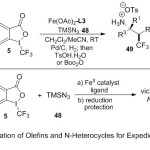 |
Scheme 15 |
Conclusion
In the last four to five years, growing proof of the single electron transfer methods from metal, inorganic salt reagents, and photoredox catalysts to out put CF3 moiety, pointing for the multilateral reductive trifluoromethylation reactions, have been widely display. In this article, the trifluoromethylation reactions allow for dynamic building of diverse C(sp2, sp3)-CF3 bonds, producing to different trifluoromethylated alkene, arene and heteroarene products with higher yields. We trust that this review can encourage chemists to perform further research task on the plan of new reagents and the employment of the old ones to improve the beneficial trifluoromethylation reactions with variety range of substrates framework.
References
- (a) I. Ojima, Fluorine in Medicinal Chemistry and Chemical Biology; Wiley: Chichester, UK, 2009;
CrossRef
(b) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2004.
CrossRef - (a) T. Nakajima, J. Fluorine Chem. 2013, 149, 104-111;
CrossRef
(b) D. Moujalled, CNS Drugs, 2016, 30(3), 227-243.
CrossRef - (a) T. Liang, C. N. T. Neumann, Ritter, Angew. Chem. Int. Ed.2013, 52(32), 8214-8264;
CrossRef
(b) T. Liu, Q. Shen, Eur. J. Org. Chem. 2012(34), 6679-6687;
CrossRef
(c) A. Studer, Angew. Chem.2012, 124(36), 9082-9090;
CrossRef
(d) T. Besset, C. Schneider, D. Cahard, Angew. Chem.2012, 124(21), 5134-5136;
CrossRef
(e) T. Besset, C. Schneider, D. Cahard, Angew. Chem. Int. Ed.2012, 51(21), 5048-5050;
CrossRef
(f) X. F. Wu, H. Neumann, M. Beller, Chem. Asian J. 2012, 7(8), 1744-1754;
CrossRef
(g) J. Nie, H. C. Guo, D. Cahard, J. A. Ma, Chem. Rev.2011, 111(2), 455-529;
CrossRef
(h) T. Furuya, A. S. Kamlet, T. Ritter, Nature, 2011, 473, 470-477;
CrossRef
(i) V. V. Grushin, Acc. Chem. Res.2010, 43(1), 160-171;
CrossRef
(j) R. J. Lundgren, M. Stradiotto, Angew. Chem.2010, 122(49), 9510-9512;
CrossRef
(k) R. J. Lundgren, M. Stradiotto, Angew. Chem. Int. Ed. 2010, 49(49), 9322-9324;
CrossRef
(l) M. Schlosser, Angew. Chem.2006, 118(33), 5558-5572;
CrossRef
(m) M. Schlosser, Angew. Chem. Int. Ed.2006, 45(33), 5432-5346;
CrossRef
(n) P. T. Nyffeler, S. G. Durn, M. D. Burkart, S. P. Vincent, C. H., Wong, Angew. Chem. 2005, 117(2), 196-217;
CrossRef
(o) P. T. Nyffeler, S. G. Durn, M. D. Burkart, S. P. Vincent, C. H., Wong, Angew. Chem. Int. Ed. 2005, 44(2), 192-212;
CrossRef
(p) K. M., Dawood, Tetrahedron, 2004, 60(7), 1435; (q) J. A., D. Ma, Cahard, Chem. Rev. 2004, 104(12), 6119-6146. - (a) K. Muller, C. Faeh, F. Diederich, Science, 2007, 317(5846),1881-1886;
CrossRef
(b) M. Hird, Chem. Soc. Rev.2007, 36, 2070-2095;
CrossRef
(c) K. L. Kirk, Org. Process Res. Dev. 2008, 12(2), 305-321.
CrossRef - H. Egami, M. Sodeoka, Angew. Chem., Int. Ed. 2014, 53(32), 8294-8308.
CrossRef - (a) T. Umemoto, Agents, Chem. Rev. 1996, 96(5), 1757-1778;
CrossRef
(b) G. K. Prakash, A. K., Yudin, Chem. Rev. 1997, 97(3), 757-786;
CrossRef
(c) T. Billard, B. R. Langlois, Eur. J. Org. Chem. 2007, (6), 891-897;
CrossRef
(d) J. Nie, H. -C. Guo, D. Ma, J. -A. Cahard, Chem. Rev. 2011,111(2), 455-529;
CrossRef
(e) O. A. Tomashenko, V. V. Grushin, Chem. Rev.,2011,111(8), 4475-4521;
CrossRef
(f) A. Studer, Angew. Chem.Int. Ed. 2012, 51(36), 8950-8958;
CrossRef
(g) Y. Ye, M. S. Sanford, Synlett, 2012, 23(14), 2005-2013;
CrossRef
(h) T. Liu, Q. Shen, Eur. J. Org. Chem. 2012, 6679-6687;
CrossRef
(i) S. B. Vallejo, A. Postigo, Coord. Chem. Rev. 2013, 257(21-22), 3051-3069;
CrossRef
(j) E. Merino, C. Nevado, Chem. Soc. Rev. 2014, 43, 6598-6608;
CrossRef
(k) S. B. Vallejo, B. Lantn˜o, A. Postigo, Chem. Eur. J. 2014, 20(51), 16806-16829.
CrossRef - (a) T. Liang, C. N. Neumann, T. Ritter, Angew. Chem., Int. Ed. 2013, 52(32), 8214-8264;
CrossRef
(b) C. P. Zhang, Q. Y. Chen, Y. Guo, J. C. Xiao, Y. C. Gu, Coord. Chem. Rev. 2014, 261, 28-72;
CrossRef
(c) C. P. Zhang, Q. Y. Chen, Y. Guo, J. C. Xiao, Y. C. Gu, Chem. Soc. Rev. 2012, 41, 4536-4559;
CrossRef
(d) J. Charpentier, N. Fruh, A. Togni, Chem. Rev. 2015, 115(2), 650-682;
CrossRef
(e) X. H. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115(2), 731-764;
CrossRef
(f) V. Bizet, R. Kowalczyk, C. Bolm, Chem. Soc. Rev.2014, 43, 2426-2438;
CrossRef
(g) E. Merino, C. Nevado, Chem. Soc. Rev.2014, 43, 6598-6608;
CrossRef
(h) C. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115(2), 765-825;
CrossRef
(i) X. Liu, C. Xu, M. Wang, Q. Liu, Chem. Rev. 2015,115(2), 683-730;
CrossRef
(j) L. Chu, F. L. Qing, Acc. Chem. Res. 2014, 47(5), 1513-1522.
CrossRef - Y. Cheng, X. Yuan, H. Jiang, R. Wang, J. Ma, Y. Zhang, S. Yua, Adv. Synth. Catal.2014, 356, 2859-2866.
CrossRef - L. L. Li, Q. -Y Chen, Y. Guo, J. Fluo. Chem. 2014, 167, 79-83.
- Y. -Y. Yu, A. R. Ranade, G. I. Georg, Adv. Synth. Catal., 2014, 356(17), 3510-3518.
CrossRef - C. Zhang, Adv. Synth. Catal. 2014, 356, 2895-2906.
CrossRef - C. Liu, B. Zhang, RSC Adv. 2015,5, 61199-61203.
- R. Beniazza, F. Molton, C. Duboc, A. Tron, N. D. McClenaghan, Chem. Commun. 2015, 51, 9571-9574.
CrossRef - S. Park, J. M. Joo, E. J. Cho, Eur. J. Org. Chem. 2015, 19, 4093-4097.
CrossRef - B. Sahoo, J. -L. Li, F. Glorius, Angew. Chem. Int. Ed. 2015, 54, 11577-11580.
CrossRef - Selected reviews on semipinacol rearrangement: (a) T. J. Snape, , Chem. Soc. Rev. 2007, 36, 1823-1842; (b) Z. -L. Song, C. -A. Fan, Y. -Q. Tu, Chem. Rev. 2011, 111(11), 7523-7556; (c) K. -D. Umland, S. F. Kirsch, Synlett 2013, 24(12), 1471-1484; (d) Z. -M. Chen, Q. -W. Zhang, Z. -H. Chen, H. Li, Y. -Q. Tu, F. -M. Zhang, J. -M. Tian, J. Am. Chem. Soc. 2011, 133(23), 8818-8821; (e) F. R. Michailidis, L. Guénée, A. Alexakis, Angew. Chem.2013, 125(35), 9436-9440; (f) Z. -M. Chen, W. Bai, S. -H. Wang, B. -M. Yang, Y. -Q. Tu, F. -M. Zhang, Angew. Chem. 2013, 125(37), 9963-9967; (g) Q. Yin, S. -L.You, Org. Lett. 2014, 16(6), 1810-1813.
- R. Tomita, T. Koike, M. Akita, Angew. Chem. Int. Ed. 2015, 54, 12923-12927.
CrossRef - N. Noto, T. Koike, M. Akita, J. Org. Chem. 2016, 81(16), 7064-7071.
CrossRef - J. -S. Lin, X. -Y. Dong, T. T. Li, N. -C. Jiang, B. Tan, Xin-Yuan X. -Y. Liu, J. Am. Chem. Soc. 2016, 138, 9357-9360.
CrossRef - B. Cui, H. Sun, Y. Xu, L. Li, L. Duan, Y. -M. Li, J. Org. Chem. 2018, 83, 6015-6024.
CrossRef - J. A. Kautzky, T. Wang, R. W. Evans, D.W.C. Mac Millan, J. Am. Chem. Soc. 2018, 140, 6522-6526.
CrossRef - J. Mestre, A. Lishchynskyi, S. Castillón, O. Boutureira, J. Org. Chem.2018, 83, 8150-8160.
- C. -L. Zhu, C. Wang, Q. -X. Qin, S. Yruegas, C. D. Martin, H. Xu, ACS Catal.2018, 8(6), 5032-5037.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









