Preparation of Two Cyclobutadiene-Steroid Derivatives. Theoretical Analysis of its Interaction with the µ, δ, and k Opiod-Receptors
Figueroa-Valverde Lauro1 , Rosas-Nexticapa Marcela2, Mateu-Armand Virginia2, Diaz-Cedillo Francisco3, Hau-Heredia Lenin1, Lopez-Ramos Maria1, Garcia-Cervera Elodia1, Pool-Gomez Eduardo1 and Cauich-Carrillo Regina1
, Rosas-Nexticapa Marcela2, Mateu-Armand Virginia2, Diaz-Cedillo Francisco3, Hau-Heredia Lenin1, Lopez-Ramos Maria1, Garcia-Cervera Elodia1, Pool-Gomez Eduardo1 and Cauich-Carrillo Regina1
1Laboratory of Pharmaco-Chemistry at the Faculty of Chemical Biological Sciences of the University Autonomous of Campeche, Av. Agustín Melgar s/n, Col Buenavista C.P.24039 Campeche Cam., México.
2Acultad de Nutrición, Universidad Veracruzana. Médicos y Odontólogos s/n, 91010, Xalapa, Veracruz. México.
3Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, D.F. C.P. 11340, México.
Corresponding Author E-mail: lauro_1999@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340601
Article Received on : 09-01-2018
Article Accepted on : 02-11-2018
Article Published : 04 Nov 2018
The objective of this investigation was to develop two cyclobutadiene-steroid derivatives (compounds 6 or 7) to evaluate its theoretical interaction on µ, d, and k opioid-receptors. The synthesis of 6 or 7 was carried out using a series of reactions which involves. 1) addition/cyclization: 2) imination, 3) etherification and 4) oxy-functionalization. Chemical structure of all compounds was confirmed using elemental analysis and NMR spectra. In addition, a theoretical analysis on the interaction of compounds 6 or 7 with µ, d, and k opioid-receptors was evaluated using a docking model. The results showed that 6 or 7 may interact with different type of amino acids residues on surface of the µ, d, and k opioid-receptors. Other data, indicated that inhibition constant (Ki) involved in the interaction of compounds 6 or 7 with k receptor was less compared with the Ki present in the interaction with µ, d, receptors. These data indicated that 1) compounds 6 or 7 show a high affinity by k-receptor; 2) the cyclobutadiene analogs are particularly interesting, because these drugs may constitute a novel therapy for pain.
KEYWORDS:Cyclobutadiene; Opioid; Receptors; Steroid Derivatives
Download this article as:| Copy the following to cite this article: Lauro F. V, Marcela R. N, Virginia M. A, Francisco D. C, Lenin H. H, Maria L. R, Elodia G. C, Eduardo P. G, Regina C. C. Preparation of Two Cyclobutadiene-Steroid Derivatives. Theoretical Analysis of its Interaction with the µ, δ, and k Opiod-Receptors. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Lauro F. V, Marcela R. N, Virginia M. A, Francisco D. C, Lenin H. H, Maria L. R, Elodia G. C, Eduardo P. G, Regina C. C. Preparation of Two Cyclobutadiene-Steroid Derivatives. Theoretical Analysis of its Interaction with the µ, δ, and k Opiod-Receptors. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52022 |
Introduction
There are several drugs for treatment of pain such as codeine. fentany 1, buprenorphine, butorphanol and others,1,2 however some these drugs can cause some adverse effects such addiction.3 respiratory depressant4, sedation, dizziness, nausea, vomiting, constipation.5 In the search new therapeutic alternatives for treatment of pain, several drugs were developed. In this sense, there is reports that a diethyl-benzamide analog have high affinity by δ-receptor.6 Additionally, a report indicates that an acetamide derivative exert an analgesic effect via k-receptor activation using a rat inodel.7 Another study showed that a k-receptor agonist (U-50,4H8) was prepared and their biological activity was e valuated in a guinea pig model.8 Other data describes the synthesis of 2-[(Acylamino)ethyl]-l,4-benzodiazepines and their interaction with k-receptor using a theoretical docking model.9 Also, a report showed that arylacetamidc and benzomorphan derivatives can act as agonists of k-receptor using a model based on pharmacophores and coupling.10 Finally, a report indicated the preparation and interaction of a piperidine analog on δ-receptor using a docking model.11 All these data indicate that some drugs may interact with different types of opioid-receptors; this phenomenon could be due to different functional groups involved in the chemical structure or to diverse protocols used. The objective of this study was synthesizing two cyclobutadiene-steroid analogs to evaluate their theoretical interaction with µ, δ, and k opioid-receptors.
Experimental
Chemical Synthesis
The nitro-progesterone was prepared using previously method reported.13 In addition, all the reagents used in this study were purchased from Sigma-Aldrich Co., Ltd. Infrared spectra (IR) were determined using KBr pellets on a Perkin Elmer Lambda 40 spectrometer.1 H and 13C NMR (nuclear magnetic resonance) spectra were recorded on a Varian VXR300/5 FT NMR spectrometer at 300 and 75.4 MHz (megahertz) in CDCl3 (deuterated chloroform) using TMS (tetramethylsilane) as an internal standard. EIMS (electron impact mass spectroscopy) spectra were determined using a Finnegan trace gas chromatography Polaris Q-spectrometer. Elementary analysis data were determined from a Perkin Elmer Ser. II CHNS/02400 elemental analyser.
NI-{1-[3-(2-Amino-ethylimino)-10,13-dimethyl-4-nitro-hexadecahydro-cyclopenta[a] phenanthren-17-yl]-ethylidene)-ethane-1,2-diamine (2)
A mixture of 1 (200 mg. 0.55 mmol), ethylenediamine (60 µl, 0.90 mmol) and boric acid (50 mg, (1.80 mmol) and 5 ml of methanol was stirred for 72 h to room temperature. The solvent of reaction product was evaporated to dryness under reduced pressure. Then, the residue was purified via crystallization with methanol:water (4:1) system; yielding 52 % of product, m,p. 215-220oC: IR (Vmax, cm-1): 3380 and 15 St). ‘H NMR (300 MHz. CDCl3) δH: 0.88 (s. 2H), 0.92 (s. 3H), 1.06-1.68 (m, 11H). 1.80 (s, 3H), 1.84-2.50 (m. l0H). 3.10 (m, 4H), 3.52 (m, 2H), 3.82 (m, 2H), 4.34 (broad, 4H), 5.14 (m, l H) ppm. 13C NMR (75.4 Hz, CDCl3) δC: 13.22, 14.52, 16.70, 21 24, 22 36, 26,42, 26.62, 29.53, 30.94. 35.76, 36, 82, 38.14, 41.00, 42.82, 43.54, 50.99, 52.13, 52.44, 52.84, 53.12, 63.16, 79.22, 156.70, 161.60 ppm. EI-MS: m/z 445.34. Anal. Calcd. for C25 H43 N5O2: C, 67.38; H, 9.73; N, l5.72; 0, 7.18. Found. C, 67.29; H. 9.68.
6-[2-(17-(1-[2-(4-Hytlroxy-hex-1-ynylamino)-ethylimino]-ethyl}-10,15-dimethyl-4-nitro-hexadecahydro-cyclopenta[a]phenanthren-3-ylideneamino)-ethylamino]-hex-5-yn-3-ol (3)
A mixture of 2 (200 mg, 0.45 mmol), 1-hexyn-3-ol (80, µl,72 mmol), Copper(11) chloride (68 mg, 0.50 mmol), 5 ml of methanol was stirred for 72 h to room temperature. Following. the residue was purified via crystallization using methanol:hexane:water (3:1:2) system; yielding 44% of product. m.p. 58-60oC; IR (Vmax, cm-1). 3400, 3322, 3310 and 1552: 1H NMR (300 MHz. CDCl3) δH: 0.88 (s, 3H), 0.90 (s, 3H), 1.00 (s, 6H), 1.06-1.35 (m, 6H). 1.40-1.46 (m, 3H), 1.52-1.54 (m, 2H), 1.60 (m, 2H), 1.64-1.67 (m, 2H), 1.80 (s, 3H), 1,82-2.50 (m, l0H), 2.60-2.66 (m, 4H), 3.20 (m, 4H), 3.32 (broad, 4H), 3.54 (m, 2H), 3.70 (m, 2H), 3.88 (m, 2H), 5.14 (m, 1H) ppm. 13C NMR (75.4 Hz, CDCI3) dH: 10.70, 13.22. 14.50, 16.70, 21.20, 22.36, 24.10, 26.62, 29.41, 29.53, 30.9 l, 35.76, 36.82, 35.17, 42.82, 43.54, 50.26, 51.00, 51.25, 52.44, 52.87, 54.14, 63.11, 75.83, 79.14, 79.22, 92.40, 156. 70, 161.60 ppm. EI-MS: m/m 637.45. Anal. Calcd. for C37H59N5O4. C, 69.67; H. 9.32; N, 10 98; O, 10.03. Found. C, 69.54; H, 9.26.
6-((2-(E)-1-((4Z,9E,11aR,13aS)-2-ethyl-11a,l3a-dimethyl-3,6,7,8,10,11,1la,1lb,12,13, l3a,14,l5,l6,16a,16b.17,18,18a.18b-icosahytlro-2H-cyclopenta[7,8] phenanthro(1,2-b][1]oxa[4,7]diazacycloundec-3-en-8-yn-14-yl)ethylidcne)amino)ethyl)amino)hex-5-yn-3-ol (4)
A mixture of 3 (200 mg, 0 31 mmol), potassium carbonate anhydrous (50 mg. 0.36 mmol) in 5 ml of dimethyl sulfoxide was stirred for 72 h to room temperature. Following, the residue was purified via crystallization from mcthanol: water (3:1) yielding 56% of product, m.p. 240-242oC: IR (Vmax, cm-1) 3400, 33 22, 3310 and 1170. 1H NMR (300 MHz. CDCl3) δH: 0 80 (s, 3H), 0.86 (s, 3H), 0.88 (s, 3 H), 0.92 (m, 1H), 1 00 (s. 3H), 1.06-1.50 (m, 9H), 1.51 (m, 2H), 1.52 (m, 2H), 1.60 (m, lH), 1.64 (m. 1H), 1.70 (m, lH), l.76 (m, 1H), 1.80 (s, 3H), 1 82-1.84 (m, 2H), 1.88 (m, lH), 1.94-2.50 (m. 6H), 2.60-2.64 (m. 2H), 3.10 (m, 2H), 3.l8-3.56 (m, 4H), 3.66 (m, 1H), 3.70 (m. 1H), 420 (m. 2H), 4.56 (m. 1H), 5.60 (broad, 3H) ppm. “C NMR (75.4 Hz, CDCls) δC: 10.74. 11.06. 13.24. 16.25, 16.70, 21.22, 23.26, 23.42, 24.09, 24.12, 26.44, 26.62, 28.92, 29.41, 31.37, 35.37, 36.69, 38.17, 42 29, 42.82, 51.25, 51.27, 52.39, 52.44, 53.65, 54.14. 56.15, 63.11, 75.83, 79.12, 79.32, 81.26, 86.38, 88.94, 92.39, 156.70, 164.60 ppm. El-MS. m/z 590.45 Anal. Calcd. for C37H58N4O2: C. 75.21. H, 9.89; N. 9.48; O, 3.42. Found. C, 75.14; H, 9 80.
5-ethyl-N-(2-(((E)-1-((4Z,9E,11aR,13aS)-2-ethyl-11a,13a-dimethyl-3,6,7,8, 10,11,11a,11b,12,13,13a,14,1S,16,16a,I6b,17,18,18a,18b-icosahydro-2H-c› clopenta[7,8)phenanthro- [1,2-b][1]oxa[4,7]diazacycloundec-3-en-8-yn-14-yl)ethylidene)amino)ethyl)-4,3-dihydrofuran-2-amine (5)
A mixture of 4 (200 mg, 0.34 mmol). Copper(II) chloride (46 mg, 0 34 mmol), 5 ml of methanol was stirred for 72 h at room temperature, Then, the mixture was purified via crystallization using methanol: hexane: water (4:2: 1) system, yielding 66 % of product. m.p. 90-92oC. IR (Vmax, cm-1) 3330, 3310 and 1172. 1HNMR (300 MHz. CDCl3) δH: 0.80 (s, 3H), 0.86 (s, 3H), 0.88 (s, 3H), 0.90 (s, 3H), 0.96-1.50 (m, 9H), 1.51 (m, 2H), 1.52-1.64 (m, 1H), 1.66 (m, 1H), 1.70 (m, 1H), 1.72 (m. 1H), 1.76 (m, 1H). 1.80 (s. 3H), 1 82-1.84 (m, 2H), 1.90 (m, l H), 1.94 (m, 1H), 2.00 (m. l H), 2.12-2.36 (m, 3H). 2.39 (m, 1H), 2.40-2.50 (m, 2H), 3 10 (m, 2H), 3.44-3.56 (m, 4H), 3.64 (m, lH), 4.22 (m, 2H). 4.56 (m, 1H), 4.60 (m, 1H). 4 74 (d, 1 H, J = 1 05 Hz), 7.32 (broad, 2H) ppm, 13C NMR (75.4 Hz. C DCl3) δC: 9.84, 1.10, 13.24, l 6.22, 16.73. 21.21, 23.26, 23.30, 23.34, 23.42, 24.09, 26.42, 26.62, 28.92, 31.37, 35.76, 35.78, 36.69, 38.17, 42.29, 42.82. 42.95, 51.27, 52.35, 52.39, 52.44, 53.65, 56.15, 63.11, 78.66, 79 32, 81.26, 86.38, 88.94, 92.94, l 55.22, 156.70, 164.60 ppm. EI-MS m./z 590.45. Anal. Calcd. for C37H58N4O2: C, 75.21; H. 9.89; N, 9.48; 0. 5.42. Found: C, 75.16; H, 9.78.
(3-ethyl-1-((2-((E)-1-((5aS,7bR,E)-17-ethyl-14-(hydroxy(phenyl)methyl)-5a,7b-dimethyl-2, 2a,2b,3,4,5,5a,6,7,7a,7b,8,9,11,12,l3,l6,17,18a,18b-icosahydro-1H-cyclobuta[h]cyclo- penta[7,8]phenanthro[1,2-b][1]oxa[4,7]diazacycloundecin-5-yl)ethylidene)amino) ethy1)amino)-2-oxabicy’clo[3.2.0]hept-Gen-I›-yI)(phenyI)methanol (6)
A mixture of 5 (200 mg, 0.34 mmol), 1-phenyl-2-propyn-1-ol (40 µl, 0.33 mmol), Copper(II) chloride (46 mg, 0.34 mmol), 5 ml of methanol was stirring for 72 h at room temperature. Then, the mixture was purified via crystallization using the methanol:hexanc (4:1) system, yielding 55% of product. m.p. 98-100oC: IR (Vmax, cm-1): 3400. 3332. 3322, 1624 and 1170: ‘H NMR (300 MHz. CDCl3) δH: 0.84 (s, 3H), 0.88 (s, 3H), 0.92 (s, 3H), 0.94-1.28 (m, 4H), 1.34 (m, 1H), 1.38-1.46 (m, 4H), 1.47 (m, 1H), 1.50-1.52 (m, 3H). 1.53 (m, 1H), 1.62 (m, 1H), 1.64-1.76 (m, 2H), 1.80 (s. 3H), 1.81 (m, 1H), 1.83-1.94 (m. 2H), 1.96 (m, 1H), 2.12-2.50 (m, 5H). 2.52 (m, 1H), 3.00-3.06 (m. 2H), 3.26 (broad, 4H), 3.34 (m, 2H), 3.36 (m, 1H), 3.44 (m, 1H), 3.54 (m. 2H), 3.90 (m, 2H), 3.96 (m, 1H), 4.66 (m, 1H), 5.18 (m, 1H), 5.60 (m, 2H). 6.06 (d. l H. J = 1.82 Hz), 6.12 (d. 1H, J = 1.33 Hz), 7.26-7.60 (m, 10H) ppm. 13C NMR (75.4 Hz. CDCl3) δC: 9.60, 10.30, 13.24, 16.25, 16.73, 21.21, 21.51, 23.42, 24 09, 26.42, 26.62, 27.92, 31.33, 31.37, 35.76, 36.69, 38.17, 39.75, 42 29, 42.82, 43.79, 44.52, 50.34, 51.27, 52.44, 52.83, 53.65, 56.98, 63.11, 75.04, 75.86, 79.62, 81.53, 82.73, 89.50, 105.98, 126.42. 126.73, 127.82, 128.45, 128.65, 128.71, 129.26, l29.34, 137.16, 138.04, 140.59, 149.29, l56.70, 164.60 ppm. EI-MS. m/z 654.57. Anal. Calcd. for C55H74N4O4: C, 77.24; H, 8.72; N, 6.55; O. 7.48. Found: C, 77.18; H, 8.66.
1-(3-ethyl-1-((2-(((E)-1-((5aS,7bR,E)-17-ethyl-14-(2-hydroxybutyl)-5a,7b-dimcthyl-2, 2a,2h,3,4,3,5a,6,7,7a,7b,8,9,l1,12,13,16,17,18a,18b-icosahydro-1H-cycIobuta[h]cyclopenta- [7,8]phcnanthro[1,2-b][1]oxa[4,7]diazacyclounidecin-5-yl)ethylidene)amino)ethyl)- amino)-2-oxabicyclo[3.2.0]hept-6-en-6-yI)butan-2-oI (7)
A mixture of 6 (200 mg. 0 34 mmol). 1-hexyn-3-ol (80 µl, 0.72 mmol), Copper(II) chloride (68 mg, 0.50 mmol), 5 ml of methanol was stirred for 72 h at room temperature. Following, the residue was purified via crystallization using the methanol;hexane:water (4:2:1) system; yielding 38 % of product, m.p. l30-132oC: IR (Vmax, cm-1): 3400. 3332. 3320 and 1172: 1H NMR (300 MHz, CDCl3) δH: 0.84 (s, 3H), 0.88 (s. 3H), 0.92 (s. 3H), 0.93 (s. 3H), 0.94 (m. 1H), 0.96 (s, 6H), 1.06 (m, 1H), 1.20 (m. 2H), I.26-1.28 (m, 2H), 1.34 (m. 2H), 1.38 (m, 2H), 1.40-1 46 (m, 4H), 1.47 (m, 1H), 1.50-1.52 (m. 3H), 1.53 (m, 1H), 1.62 (m, 1H), 1.64-1 76 (m, 2H), 1.80 (s. 3H). 1.81 (m, 1H), 1.82-1.83 (m, 2H), 1.93 (m, 1H), 1.94-2.12 (m. 2H), 2.22 (m, 1H), 2.28 (m, 1H), 2.30 (m, 1H), 2 36-2.38 (m. 2H), 2.42 (m. 1H), 2.48 (m, 1 H), 2.50 (m, 1H), 2.52 (m, 1 H), 2.96 (broad, 4H), 3.00-3.06 (m, 2H), 3.22 (m, 1H), 3.26 (m, 1H), 3.34 (m, 2H), 3.44 (m, 1H), 3.53 (m, 1H), 3.54 (m, 2H), 3.90 (m, 2H), 3.96 (m, 1H), 4.66 (m, 1H), 5.86 (d, 1H, J = 1.82 Hz), 6.46 (d, 1H. J = 1.33 Hz) ppm. 13C NMR (75.4 Hz. CDCl3) δC: 9.56, 9. 95, 10 30, 13.24, 16.25, 16. 73, 21.21, 23.42, 24.09, 26.42, 26.62, 27.92, 29.22, 31.23, 31.37, 33.91, 35.76, 36.69, 38.17, 39.75, 42.29, 42.82, 43.79, 44.32, 44.52, 46.32, 51.27, 5 l.36, 52.44, 52.83, 53.65, 56.98, 63.11, 71.02, 74.04, 75 08, 8l.53, 82.73, 87,86, 104 05, 126.34, 126.42, 131.11, 133.82, 146.34, 156.70, 164.60 ppm, EI-MS. m/z 756.60. Anal. Calcd. for C49H 78N4O4: C, 74.76; H, 9.99; N, 7.12; O, 8. l3. Found. C. 74.70: H, 9.90.
Evaluation Of Physicochemical Parameters From Compounds 2-7
Physicochemical factors of compounds 2 to 7 such as lipophilicity degree (LogKow). HBD (hydrogen bond donor groups) and HBA (hydrogen bond acceptor groups), TPSA (topological polar surface area) and number of rotatable bonds (RB) were evaluated using a previously methods reported.13,14
Theoretical Evaluation
The interaction between compounds 6 or 7 with opioid-receptors was determinate using a Dockingserver.15,16 In addition, the interaction of compounds 6 or 7 were carried out using a theoretical model for µ (4DKL),17 δ (4RWA)18 and k (4DJH)19 opioid-receptors.
Toxicity Analysis
Theoretical toxicity of compounds 6 or 7 was determined using PASS online software.20
Results and Discussion
Chemical Synthesis
The cyclobutadiene-steroid derivatives (compound 6 and 7) were synthesized using some chemical strategies:
Preparation of an Imino-Steroid Derivative
There are some reports that shown the synthesis of several imino analogs; however, the protocols require special conditions.21,22 The first stage was achieved by reaction of a nitro-progesterone (1) with ethylenediamine (Figure 1) using boric as catalyst to synthesis of an imino-steroid analog (2); it is noteworthy that boric acid did not require special conditions.23 The 1H NMR spectra of 2 shows bands at 0.88-0.92 ppm for methyl groups bound to steroid nucleus: at 1.80 ppm for methyl bound to imino group: at l.06-1.68, 1.84-2.50 and 5.14 ppm for steroid moiety; at 3.10-3.82 ppm for methylene groups bound to both amino and imino groups: at 4.34 ppm for amino groups. The 13C NMR spectra showed chemical shifts at 13.22-14.52 ppm for methyl groups bound to steroid nucleus; at 16.70 ppm for methyl group bound to imino group: at 21.24-38. 53.12 and 63.16-79 22 ppm for steroid moiety: at 41.00-52.84 ppm for methylene groups bound to both amino and imino groups; at 156.70-161.60 ppm for imino groups. Finally, the mass spectrum shown a molecular ion at 445.34.
Preparation of Hvdroxy-Hexynylamino-4-Nitrosteroid Derivative
Several studies showed the synthesis of some hexynyl-amino analogs by addition of amine groups to alkyne-derivatives using different reagents such as Cu/DMSO24 and Ru-Cp25. In this investigation, the compound 2 reacted with 1-hexyn-3-ol (Figure 1) in presence of Copper(II) to form a hydroxy-hexynylamino-4-nitrostcroid derivative (3). The 1H NMR spectra of 3 display several signals at 0.88-0.92 ppm for methyl groups bound to steroid nucleus: at 1.00 ppm for methyl group of the arm which was bound to hydroxyl group; at 1.80 ppm for methyl bound to imino group: at 1.06-1.38, l.52-1.54, 1.64-1.67, 1.82-2 50 and 5.14 ppm for steroid moiety; at 1.40-I.46, 1.60, 3.70 and 2 60-2.66 ppm for methylene groups bound to hydroxyl and alkyne groups: at 3.32 ppm for both amino and hydroxyl groups: at 3.20. 3.54 end 3.S6 ppm for methylene groups binding to imino and amino groups. The 13C NMR spectrum showed several signals at l0.70 ppm for methyl group of the arm linked to hydroxyl group; at 13.22-14. 50 ppm for methyl groups binding to steroid nucleus: at 16.70 ppm for methyl group linked to imino group; at 21.20-22 36, 26.62, 29.53, 43 54, 51 00, 52.44-52.57, 63.11 and 79.22 ppm for steroid moiety, at 24 10, 29 41 and 75.83-79.14 ppm for methylene groups binding to hydroxj1 and alkyne groups: at 50.26, 51.25 and 54.14 ppm for methylene groups linked to both imino and amino groups: at 92.40 ppm for alkyne group: at 156.70-161.60 ppm for imino groups. The mass spectrum showed a molecular ion at 637.45.
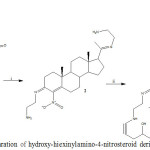 |
Figure 1: Preparation of hydroxy-hiexinylamino-4-nitrosteroid derivative (3). |
Reaction of 4-nitroprogesterone (l) with ethylenediamine (i) to form an imino-steroid analog (2). Then, 2 reacted with 5-hexyn-3-ol (ii) to synthesis of 3.
Synthesis of An Ether-Steroid Derivative (4)
There are several protocols to preparation of ether which use some reagents such as methoxy analogs,26 fluoride ion,27 D-glucose,28 sodium phenoxide,29 palladium(II),30 dimethyl sulfoxide/potassium carbonate31 and others. In this study, an ether derivative (compound 4) was prepared by the reaction of 3 with 2-hydroxy-l-naphthaldehyde (Figure 2) in presence of dimethyl sulfoxide. The ‘ H NMR spectrum of 4 display bands at 0.80- 0.86 ppm for methyl groups binding to steroid nucleus: at 1.00 ppm for methyl group of arm linked to hydroxyl group: at 1.80 ppm for methyl linked to imino group. at 0.88 ppm for methyl group of arm binding to l-Oxa-4.7-diaza-cycloundec-3-en-8-yne ring: at 0.92, 1.06-1.50, 1.52, 1.64-1.76, 1.82-1, 84, 1.94-2.50 and 3.66 ppm for steroid moiety at 1.51. 3.10 and 4.20-4. 56 ppm for 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 1.60, 2.60-2.64 and 3 70 ppm for methylene groups linked to hydroxyl and alkyne groups; at 1.70 and 1.60 ppm for methylene group binding to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 3.18-3.56 ppm for methylene groups binding to both imino and amino groups; at 5.00 ppm for hydroxyl and amino groups. The 13C NMR spectrum showed several signals at 10.70 ppm for methyl group of arm binding to hydroxyl group: at 13.22-16.25 ppm for methyl group linked to steroid nucleus. at 11.06 ppm for methyl group of arm binding to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 16.70 ppm for methyl group binding to imino group: at 21.22-23.42, 24.09, 26.44-26.62, 3l.37-42.82, 52.44-53.65 and 63.11 ppm for steroid moiety; at 23.26, 52.39. 56.15 and 1.26-88.94 ppm for 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 24.12, 29.41 and 75.83 ppm for methylene groups linked to hydroxyl and alkyne groups: at 28.92 ppm for methyl group of arm bound 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 51.25 and 54.14 ppm for methylene groups bound to imino and amino groups; at 79.12. 79.32 and 92.39 ppm for alkyne groups, at 156.70-161.60 ppm for imino groups. Finally, the mass spectrum of 5 showed a molecular ion at 590.45.
Preparation of Furanyl-steroid tIcrii»alive (5)
Analyzing the chemical structure of 4 and some reports which indicate that several metals can catalyze oxy -functionalization of alkynes32. Therefore, in this investigation 4 was reacted with Cooper(II) to form the compound 5 (Figure 2). The ‘H NMR spectra of 5 display several signals at 0.80-0.86 ppm for methyl groups binding to steroid nucleus: at 0.90 ppm for methyl group of arm linked to dihydrofuran ring: at 1.80 ppm for methyl binding to imino group: at 0.88 ppm for methyl group of arm bound to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 0.96-1 50, 1.54-1.64, 1.76, 1.82-1.84, 1.94, 2.12-2.36, 2.40-2.50 and 3.64 ppm for steroid moiety; at 1.51, 3.10 and 4.22-4.56 ppm for 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 1.66 and 1.72 ppm for dihydrofuran ring; at 1.70 and 1.90 ppm for methylene group binding to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 2.00, 2.39 and 4.60-4.74 ppm for dihydrofuran ring: at 3.44-3.56 ppm for methylene groups linked to both imino and amino groups; at 7.32 ppm for amino groups, The 13C NMR spectrum showed several signals at 9.84 ppm for methy1 group of arm binding to dihydrofuran ring; at 11.10 ppm for methyl group of arm linked to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 13.22-16.22 ppm for methyl groups bound to steroid nucleus; at 16.73 ppm for methyl group bound to imino group; at 21.21, 26.62, 31.37-35.76, 36.69-42.82, 51.27, 52.44-53.65 and 63.11 ppm for steroid moiety : at 23.26, 52,39, 56.15, 81.26 and 88.94 ppm for 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 23.34 ppm for methylene binding to dihydrofuran ring; at 28.92 ppm for methylene bound to 1-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring: at 35.78, 78.66 and 92.94-155.22 ppm for dihydrofruran ring; at 42.95 and 52.35 ppm for methylene groups bound to both imino and amino groups, at l56.70-164.60 ppm for imino groups. Finally, the compound 5 display a molecular ion at 590.45.
Preparation of Cyclobutene-Steroid Derivatives (Compounds 6 Or 7)
There are some reports which shown the [2+2] cycloaddition of alkene with alkyne groups and cycloaddition of alkyne groups to alkyne derivatives using metals as catalyst.33,34 Therefore in this study 5 was reacted with l-plienyl-2-prpyn-1-ol or 1-hexvn-3-ol to form 6 or 7 in presence of Copper(II) as catalyst (Figure 2). The ‘H NMR spectra of 6 show several bands at 0.84-0.88 ppm for methyl groups binding to steroid nucleus: at 0 93 ppm for methyl group of arm linked to 2-oxa-bicyclo(3.2.0]hept-6-cnc ring: at 1.80 ppm for methyl bound to imino group: at 0.92 ppm for methyl group of arm binding to l-Oxa-4.7- diaza-cycloundec-8-yne ring: at 0.94-1.28, 1.38-1.46, 1.50-1.52, 1.64-l.76, 1.83, 2.12-2.50 and 3.44 ppm for steroid moiety, at 1.34, 3.34, 3.90 and 4 66 ppm for l-Oxa-4, 7-diaza-cycloundec-3-en-8-yne ring, at 1.47 and 1.53 ppm for methylene group binding to l-Oxa-4,7-diaza-cycloundec-3-en-8-yne ring: 1.96, 2.52, 3.36, 3.96 and 6.12 ppm for 2-oxa-bicy- clo(3.2.0]hept-6-ene ring: at 3,00-3.06 and 3.54 for methylene groups linked to both imino and amino groups; at 7.32 ppm for amino groups; at 3.26 for both amino and a hydroxyl groups: at 5.18 ppm for methylene figroup bound to both phenyl and hydroxyl groups, at 5.60 ppm for methylene binding to both cyclobutadiene ring and phenyl group: at 6.06 ppm for cyclobutadiene ring. at 7.26-7.60 ppm for phenyl groups. The 13C NMR spectrum display several signals at 9.60 ppm for methyl group of arm bound to l -Oxa-4.7-diaza-cyc1oundec-3-en-8-yne ring; at 27.92 ppm for methylene group linked to 2-oxa-bicyclo[3.2 0]hcpt-6-cnc ring; at 10.30 ppm for methyl group of arm a bound to 2-oxa-bicyclo[3.2.0]hept-6-ene ring, at 13.24-16.25 ppm for methyl groups binding to steroid nucleus; at 16.73 ppm for methyl group bound to imino group: at 21 21-26 62, 31.37-38.17, 42.29-42.82, 51.27-52.44, 53.65 and 63.1l ppm for steroid moiety; at 29.92 ppm for methylene bound to 2-oxa-bicyclo[3.2.0]hept-6-ene ring; at 31.33, 50.34, 75.86, 89.50, 129.26 and 149.59 ppm for 2-oxa-bicyclo [3,2.0]hcpt-6-ene ring; at 39.75, 43.79, 56 98 and 81.53-82.73 ppm for 2-oxa-bicyc 1o (3.2.0] hept-6-ene ring; at 44.52 and 52.53 ppm for methylene groups bound to both amino and imino groups: at 79.62 ppm for methylene group binding to both phenyl group and 2-oxa-bicyclo[3.2.0]hept-6-ene; at l05.92, 126.73 and 129.34-137.16 ppm for cyclobutadiene ring; at 126.43, 127.82-l28.71 and 138.04-140.59 ppm for phenyl groups: at 156.70-164.60 ppm for imino groups. Additionally, the compound 6 showed a molecular ion at 854.57.
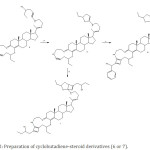 |
Figure 2: Preparation of cyclobutadiene-steroid derivatives (6 or 7). |
Etherification of 3 using DMSO/K2CO3 (iii) to synthesis of an ether-steroid analog (4). Then 4 reacted with Cooper(II) to formation of a Furanyl-steroid derivative (5). Finally, 6 or 7 were synthesized via reaction of 5 with 1-phenyl-2-propyn-1-ol (v) or 1-hexyn-3-ol (vi).
Finally, the signals found of the ‘H NMR spectrum for 7 showed several bands at 0.84-0.88 ppm for methyl groups binding to steroid nucleus: at 0.92 ppm for methyl group of arm bound to 2-oxa-bicyclo [3.2.0] hept-6-ene ring; at 1.80 ppm for methyl linked to imino group; at 0.92 ppm for methyl group of arm bound to 1-oxa-4.7- diaza-cycloundec-3-en-8-yne ring; at 0.94, 1.06, 1.26-1.28, 1.40-1.46, 150-1.52, 1.64-1.76, 1.82-1.83, 1.94-2.12, 2.28, 2.36, 2.50 and 3.44 ppm for steroid moiety; at 1.34. 3.34. 3.90 and 4.66 ppm for 1-Oxa-4,7-diaza-cy’cloundec-3-en-8-yne ring; at 1.20, 1.38, 2.22, 2.30, 2.42, 2.52, 3.22 and 3.53 ppm for methylene groups of arm binding to both cyclobutadiene ring and hydroxyl group; at 1.47 and 1.53 ppm for methylene group bound to l -Oxa-4.7-diaza-cycloundec-3-en-8-yne ring; at 1.62 and 1 8 l ppm for methylene bound to 2-oxa-bicyclo[3. 2.0]hcpt-6-enc ring: at 1.93, 2.48. 3.26, 3.96 and 6.46 ppm for to 2-oxa-bicyclo[3.2.0]hept-6-ene ring; at 2.96 ppm for both amino and hydroxyl groups; at 3.00- 3.06 and 3.54 ppm for methylene groups linked to both amino and imino groups; at 5 86 ppm for cyclobutadiene ring. The 13C NMR spectrum display several signals at 9.56 ppm for methyl group of arm bound to 1-Oxa-4,7-diaza-cycloundec-3-en-8-yne ring; at 10.30 ppm for methyl group of arm bound to 2-oxa-bicyclo[3.2.0]hept-6-ene ring; at 13.24-16.25 ppm for methyl groups binding to steroid nucleus: at 16.73 ppm for methyl group bound to imino group; at 9.95 ppm for methyl group of arm bound to both cyclobutadiene ring and hydroxyl group; at 21.21 -26.62, 31.37, 35.76-35. 17, 42.29-42.82, 51.27, 52.44, 53.65 and 63.11 ppm for steroid moiety; at 27,92 ppm for methylene group binding to 2-oxa-bicycIo [3.2 0]hept-6-ene ring; at 31.33, 51.36,. 75.08, 87.86, l26.34, and 146.34 ppm for 2-oxa-bicyclo [3.2.0] hept-6-ene ring; at 33.9 l, 44.30, 46.32 and 71.02-74.04 ppm for methylene groups of arm bound to both cyclobutadiene ring and hydroxyl group; at 44.52 and 52.83 ppm for methylene groups bound to both amino and imino groups; at 156.70-164.60 ppm for imino groups. Finally, the compound 7 display a molecular ion at 786.60.
Physicochemical Parameters
Analyzing some reports, which indicate that some physicochemical factors of several drugs such as hydrogen bond donor groups (HBD) and hydrogen bond acceptor groups (HBA), topological polar surface area (TPSA) and number of rotatable bonds (RB) are used to predict the biological activity of some compounds in different theoretical models35-37; therefore, in this study these physicochemical parameters (Table 1) were evaluated using the Schrodinger Software.38 The results indicate that both HBA, HBD values were <5, these data indicate that 7 could be well absorbed, such happening with other type of compounds.39 Another result showed that polarity for 7 was higher compared the compounds 2 to 6; here it is noteworthy some studies indicate that this physicochemical parameter could condition the ability of several drugs to penetrate the blood-brain barrier affinity and exhibit biological activity on nervous central system.40
Table 1: Physicochemical parameters of compounds 2 to 7.
| Compound | MW | HBA | HBD | RB | TPSA | Polar |
|
2 |
797.24 | 4 | 2 | 14 | 107.04 | 92.44 |
|
3 |
450.68 | 1 | 1 | 8 | 122.63 | 50.83 |
|
4 |
650.99 | 3 | 3 | 20 | 135.11 | 74.12 |
|
5 |
602.97 | 2 | 1 | 11 | 77.58 | 71.47 |
|
6 |
600.96 | 2 | 0 | 8 | 66.58 | 70.77 |
|
7 |
877.37 | 4 | 4 | 12 | 107.04 | 109.91 |
Bond donor groups (HBD) and hydrogen bond acceptor groups (HBA), topological polar surface area (TPSA) and number of rotatable bonds (RB).
Table 2: Physicochemical parameters LogP and π of compounds 1-3.
| Compound | Fragment | Value |
| 1 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C(=O)- [aliphatic carbon attach]-NO2 [nitro aliphatic attach]-tert Carbon [3 or more carbon attach]Fused aliphatic ring unit correctionC-(NO2)-CO- structure correction
Equation constant π LogKow |
1.64193.92882.1684-3.1172-0.81320.5352-2.05261.0000
0.2290 -0.52 3.5203 |
| 2 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]-NH2 [aliphatic attach]-NO2 [nitro aliphatic attach]-tert Carbon [3 or more carbon attach]-N=C [aliphatic attach]
Fused aliphatic ring unit correction >C=N-C [cyclic-type imine, ali carbor att] Equation constant π LogKow |
1.64.195.89322.16841.9446-2.8296-0.81320.5352-0.0020
-2.0526 -1.5500 0.2290 1.6446 5.1649 |
|
3 |
-CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]#C [acetylenic carbon]-OH [hydroxyl, aliphatic attach]-NH2 [aliphatic attach]-NO2 [nitro aliphatic attach]
-tert Carbon [3 or more carbon attach] -N=C [aliphatic attach] multi-alcohol correction Fused aliphatic ring unit correction >C=N-C [cyclic-type imine, ali carbor att] Equation constant π LogKow |
2.73657.85762.89121.94460.5336-2.8172-2.9924-0.8132
0.5352 -0.0020 0.4064 -2.0526 -1.5500 0.2290 1.7418 6.9067 |
On the other hand, also another parameters such as logP and π41 were used to delineate the structural chemical requirements of compounds 1 to 7,; it is noteworthy that logP can be used to determinate lipophilicity degree of either molecule, therefore, in this study these parameters were calculated. The results shown in the Tables 3 and 4 indicate that aliphatic carbons (-CH3, -CH2 and aromatic carbon) involved in the compound 6 contribute to increase the lipophilicity compared with the compounds 2-5 and 7. All this data suggest that changes in the degree of lipophilicity depend of structural chemical characteristic of compounds studied.
Table 3: Physicochemical parameters LogP and π of compounds 4 and 5.
| Compound | Fragment | Value |
| 4 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]#C [acetylenic carbon]-OH [hydroxyl, aliphatic attach]-O- [oxygen, aliphatic attach]-NH- [aliphatic attach]
-tert Carbon [3 or more carbon attach] -N=C [aliphatic attach] Fused aliphatic ring unit correction Equation constant π LogKow |
2.73657.95762.89121.94460.5336-1.4086-1.2566-2.9924
0.5352 -0.0020 -2.7368 0.2290 1.4246 8.3313 |
| 5 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]=CH- or =C< [olefin carbon]-O- [oxygen, aliphatic attach]-NH- [aliphatic attach]-tert Carbon [3 or more carbon attach]
-N=C [aliphatic attach] Fused aliphatic ring unit correction Equation constant π LogKow |
2.73657.85762.89121.94461.5344-2.5132-2.99240.5352
-0.0020 -2.7368 0.2290 2.5774 9.4841 |
Table 4: Physicochemical parameters LogP and π of compounds 6 and 7.
| Compound | Fragment | Value |
| 6 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]=CH- or =C< [olefin carbon]-OH [hydroxyl, aliphatic attach]-O- [oxygen, aliphatic attach]-NH- [aliphatic attach]
Aromatic carbon -tert Carbon [3 or more carbon attach] -N=C [aliphatic attach] multi-alcohol correction -C-O structure correction Fused aliphatic ring unit correction Equation constant π LogKow |
2.73657.85763.97542.91692.3016-2.8172-2.5132-2.9924
3.5280 0.5352 0-0020 0.4064 0.5494 -2.3947 0.2290 4.8355 14.3165 |
| 7 | -CH3 [aliphatic carbon]-CH2 [aliphatic carbon]-CH [aliphatic carbon]-C [aliphatic carbon – No H, no tert]=CH- or =C< [olefin carbon]-OH [hydroxyl, aliphatic attach]-O- [oxygen, aliphatic attach]-NH- [aliphatic attach]
-tert Carbon [3 or more carbon attach] -N=C [aliphatic attach] multi-alcohol correction -C-O structure correction Fused aliphatic ring unit correction Equation constant π LogKow |
3.83119.82203.97542.91692.3016-2.8172-2.5132-2.9924
0.5352 -0.0020 0.4064 0.5494 -2.3947 0.2290 4.3634 13.8475 |
Docking Evaluation
Some studies suggest that some compounds exert their biological activity via opioid receptors; therefore, in this study was evaluated that two cyclobutadiene-steroid derivatives (compound 6 or 7) could interact with opioid receptors such as µ (4DKL),16 δ (4RWA)17 and k (4DJH)18 using a docking model13. The results (Figure 3) show the possible interaction of compound 6 with several aminoacid residues involved in the structure of µ opioid receptor such as Gln124, Asn127, Tyr128, Asp147, Tyr148, Met151, Thr218, Lys233, Cys210, Val236, Ile296, His297, Val300, Trp318, His319, Ile322, Tyr326. In addition, theoretical interaction of compound 7 with this same opioid-receptor (Figure 6) shown that could bind with several aminoacid residues such as Asp147, Tyr148, Met151, Thr218, Leu219, Lys233, Val236, Phe237, His297, Val300, Ile301, Lys303, Glu310, Gln314, Thr315, Trp318, His319, Ile322.
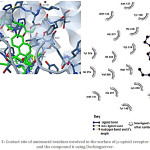 |
Figure 3: Contact site of aminoacid residues involved in the surface of µ-opioid receptor (4DKL) and the compound 6 using Docking server. |
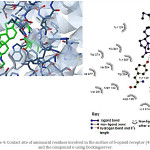 |
Figure 4: Contact site of aminoacid residues involved in the surface of δ-opioid receptor (4RWA) and the compound 6 using Docking server. |
Also, was evaluated the possibility of that cyclobutadiene-steroid derivative may interact with δ opioid-receptor. the results (Figure 4) showed the interaction of 6 with several amino acid residues such as Tyr109, Asp128, Tyr129, Met132, Leu200, Asp210, Lys214, Trp274, Ile277, Val281, Trp284, His301, Ile304, and Tyr308 involved in the structure of δ opioid receptor. In addition, theoretical asses indicated the interaction of several aminoacid residues of this receptor with the compound 7 such as Gln105, Lys108, Tyr109, Asp128, Tyr129, Val197, Leu200, Asp210, Lys214, Val281, Trp284, Thr285, Val297, Leu300 and Tyr308 (Figure 7).
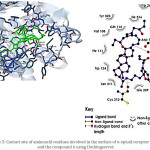 |
Figure 5: Contact site of amino acid residues involved in the surface of k-opioid receptor (4DJH) and the compound 6 using Docking server. |
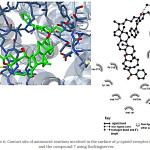 |
Figure 6: Contact site of aminoacid residues involved in the surface of µ-opioid receptor (4DKL) and the compound 7 using Docking server. |
Finally, the possible interaction of compounds 6 or 7 with κ-opioid receptor was evaluated. The interaction of 6 with κ-receptor involved several aminoacid residues (Figure 5) such as Val108, Thr111, Gln115, Trp124, Asp138, Glu209, Cys210, Ser211, Tyr219, Lys227, Trp287, Ile290, Ile294, Tyr312, Ile316 and Tyr320. Additionally, the interaction of 7 with κ-receptor involves the following aminoacid residues (Figure 8) such as Thr111, Phe114, Gln115, Val118, Trp124, Val134, Leu135, Asp138, Tyr139, Met142, Glu209, Cys210, Ser211, Tyr219, Asp223, Met226, Lys227, Trp287, Ile290, Ile294, Tyr312, and Ile316. All this data indicates that compound 6 or 7 interact in a manner different with aminoacid residues on surface of µ, δ and k opioid-receptors. This phenomenon could involve other type of intramolecular interactions due to changes in the energy levels.
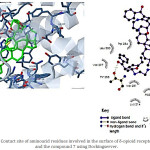 |
Figure 7: Contact site of aminoacid residues involved in the surface of δ-opioid receptor (4RWA) and the compound 7 using Dockingserver. |
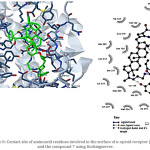 |
Figure 8: Contact site of amino acid residues involved in the surface of κ-opioid receptor (4DJH) and the compound 7 using Docking server. |
Thermodynamic Parameters
Some studies indicate that thermodynamic parameters are evidences for confirming the interaction drug-protein42. in this study a theoretical evaluation ii as carried out on some thermodynamics parameters such as free energy of binding, electrostatic energy, total intermolecular energy, vd W + H bond + desol energy and inhibition constant. The results showed differences in the intramolecular energy involved in the interaction for compound 6 (Table 5) or 7 (Table 6) with µ, δ and κ opioid-receptors. In addition. other data shown different inhibition constants involved in the interaction or compounds 6 or 7 (Ki = 11.23; Ki = 0.20 with κ-opioid receptor. However, the values of these inhibition constants were lower compared with the Ki involved in the interaction of compounds 6 or 7 with µ and δ-opioid receptors; these data are interesting, which implies a higher interaction of 6 or 7 with the k-opioid receptor (which is related to analgesia effect.43) This phenomenon opens the possibility that these compounds could be evaluated experimentally in some biological system. In addition, it is important to perform some toxicity studies to evaluate if there are any side effects in the biological activity of the compounds involved in this study.
Table 5: Intrramolecular parameters involved between of interaction of the compound 6 and µ, δ and κ-pioid receptors.
| Opioid receptor | Est. Free Energy of Binding (Kcal/mol) | Inhibition Constant (Ki, mM) | vdW + Hbond desolv Energy (Kcal/mol) | Electrostatic Energy (Kcal/mol) | Total Intermol. Energy (Kcal/mol) | Surface |
| µ (4DKL)δ (4RWA)κ (4DJH) | -4.15 | 901.28 | -8.39 | -0.04 | -8.43 | 1373.3 |
| -4.33 | 673.31 | 0.8 | -0.55 | -0.86 | 1398.4 | |
| -2.66 | 11.23 | -4.1 | -0.84 | -4.94 | 1534.9 |
Table 6: Intrramolecular parameters involved between of interaction of the compound 7 and µ, δ and κ-pioid receptors.
| Opioid receptor | Est. Free Energy of Binding (Kcal/mol) | Inhibition Constant (Ki, mM) | vdW + Hbond desolv Energy (Kcal/mol) | Electrostatic Energy (Kcal/mol) | Total Intermol. Energy (Kcal/mol) | Surface |
| µ (4DKL)δ (4RWA)κ (4DJH) | -3.73 | 1.84 | -7.07 | 0.52 | -6.55 | 1331.6 |
| -9 | 254.19 | 9.89 | -0.39 | -10.28 | 1520.9 | |
| 119.06 | 0.2 | 91.8 | -0.47 | 91.34 | 1600.6 |
Theoretical Analysis of Toxicity
Analyzing the premise above mentioned. in this study also was evaluated the possible toxicity induced by the compounds 6 or 7 using the GUSAR software.44 The results (Table 7) showed that toxicity could be higher via intraperitoneal administration of compounds 6 or 7 compared to the other type of administration routes such as intravenous, oral and subcutaneous.
Table 7: Theoretical analyses of LD50 of compounds 6 or 7 using GUSAR software.
| Compounds | Rat IP, LD50 (Log10; mmo/Kg) | Rat IV, LD50 (Log10; mmo/Kg) | Rat Oral LD50 (Log10; mmo/Kg) | Rat SC, LD50(Log10; mmo/Kg) |
| 6 | -0.465 out of AD | -1.640 in AD | 0.132 in AD | -1.309 in AD |
| 7 | -0.109 in AD | -1.678 in AD | 0.184 in AD | -1.190 in AD |
Conclusions
In conclusion. all these data indicate that 1) the compounds 6 or 7 show a high affinity by κ-receptor. 2) the cyclobutadiene-steroid derivatives are particularly interesting, because these drugs may constitute a novel therapy for treatment of pain.
References
- Mao, J. Pain. 2002, 100(3), 213-217.
- Ling, W.; Mooney, L.; Hillhouse, M. Drug Alcohol Rev. 2011, 30(3), 300-305.
CrossRef - Cepeda, M.; Alvarez, H.; Morales, O.; Carr, D. Pain. 2004, 107(1-2), 41-46.
CrossRef - Benedetti, F.; Amanzio, M.; Baldi, S.; Casadio, C.; Maggi, G. Eur. J. Neur. 1999, 11(2), 625-631.
- Buenaventura, R.; Adlaka, R.; Sehgal, M. Pain physician. 2008, 11, S105-S120.
- Chen, X.; Adams, J.; Geller, E.; DeRiel, J.; Adler, M.; Liu-Chen, L. Eur. J. Pharmacol. 1995, 275(1), 105-108.
CrossRef - Clark, C.; Halfpenny, P.; Hill, R.; Horwell, D.; Hughes, J.; Jarvis, T. J. Med. Chem. 1988, 31(4), 831-836.
CrossRef - Vonvoigtlander, P.; Lahti, R.; Ludens, J. J. Pharmacol. Exp. Ther. 1983, 224(1), 7-12.
- Cappelli, A.; Anzini, M.; Vomero, S.; Menziani, M.; De Benedetti, P.; Sbacchi, M. J. Med. Chem. 1996, 39(4), 860-872.
CrossRef - De Ranter, C.; Verlinde, C.; Blaton, N.; Peeters, O. Neuropeptides. 1984, 5(1), 209-212.
CrossRef - Largent, B.; Gundlach, A.; Snyder, S. J. Pharmacol. Exp. Ther. 1986, 238(2), 739-748.
- Bowers, A.; Sánchez, M.; Ringold, H. J. Am. Chem. Soc. 1959, 81(14), 3702-3706.
CrossRef - Sabljić, A.; Güsten, H.; Verhaar, H.; Hermens, J. Chemosphere. 1995, 31(11-12), 4489-4514.
CrossRef - Niwa, T. J. Chem. Inf. Comp. Sci. 2003, 43(1), 113-119.
CrossRef - Macindoe, G.; Mavridis, L.; Venkatraman, V.; Devignes, M.; Ritchie, D. Nucleic acids Res. 2010, 38(suppl.2), W445-W449.
CrossRef - Karaca, E.; Melquiond, A.; De Vries, S.; Kastritis, P.; Bonvin, A. Mol. Cell. Prot. 2010, 9(8), 1784-1794.
CrossRef - Rajani, V.; Nagababu, S.; Kalyani, N.; Priya, G.; Manasa, R. Int. J. Res. Pharm. Sci. 2016, 6(3), 291-295.
- Ibrahim, M.; Hassan, A. Protein J. 2018, 1-13.
- Goldfeld, D.; Murphy, R.; Kim, B.; Wang, L.; Beuming, T.; Abel, R.; Friesner, R. J. Phys. Chem. B. 2014, 119(3), 824-835.
CrossRef - Filimonov, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Druzhilovskii, D.; Pogodin, P.; Poroikov, V. Chem. Heter. Comp. 2014, 50(3), 444-457.
CrossRef - Ottana, R.; Maccari, R.; Barreca, M.; Bruno, G.; Rotondo, A.; Rossi, A.; Vigorita, M. Bioorg. Med. Chem. 2005, 13(13), 4243-4252.
CrossRef - Liu, H.; Lieberzeit, Z.; Anthonsen, T. Molecules. 2000, 5(9), 1055-1061.
CrossRef - Lauro, F.; Francisco, D.; Marcela, R. Lett. Org. Chem. 2015, 12(6), 394-401.
CrossRef - Meldal, M.; Tornøe, C. Chem. Rev. 2008, 108(8), 2952-3015.
CrossRef - Mitsudo, T.; Naruse, H.; Kondo, T.; Ozaki, Y.;Watanabe, Y. Ang. Chem. Inter. Ed. Eng. 1994, 33(5), 580-581.
CrossRef - Feutrill, G.; Mirrington, R. N. Australian J. Chem. 1972, 25(8), 1719-1729.
CrossRef - Clark, J. Chem. Rev. 1980, 80(5), 429-452.
- Bakó, P.; Bajor, Z.; Toke, L. J. Chem. Soc., Perkin Trans.1. 1999, (24), 3651-3655.
CrossRef - McKillop, A.; Fiaud, J.; Hug, R. Tetrahedron. 1974, 30(11), 1379-1382.
CrossRef - Roenn, M.; Bäckvall, J.; Andersson, P. Tetrahedron Lett. 1995, 36(42), 7749-7752.
CrossRef - Lauro, F.; Francisco, D.; Elodia, G.; Marcela, R. Int. J. Clin. Exp. Med. 2015, 8(8), 12041.
- Liu, B.;De Brabander, J. Org. Lett. 2006, 8(21), 4907-4910.
CrossRef - Schore, N. Chem. Rev. 1988, 88(7), 1081-1119.
CrossRef - Yamamoto, Y.; Arakawa, T.; Ogawa, R.; Itoh, K. J. Am. Chem. Soc. 2003, 125(40), 12143-12160.
CrossRef - Craik, D.; Fairlie, D.; Liras, S.; Price, D. Chem. Biol. Drug Des. 2013, 81(1), 136-147.
CrossRef - Cheng, A.;Merz, K. J. Med. Chem. 2003, 46(17), 3572-3580.
CrossRef - López-Vallejo, F.; Giulianotti, M.; Houghten, R.; Medina-Franco, J. Drug Dis. Today. 2012, 17(13-14), 718-726.
CrossRef - Sudhakaran, S.; Calvin, J.; Amy, G. Chemosphere. 2012, 87(2), 144-150.
CrossRef - Bickerton, G.; Paolini, G.; Besnard, J.; Muresan, S.; Hopkins, A. Nature Chem. 2012, 4(2), 90.
CrossRef - Subramanian, G.; Kitchen, D. J. Computer-aided Mol. Design. 2003, 17(10), 643-664.
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Chem. Pharm. Bull. 1994, 42(4), 976-978.
CrossRef - Ross, P.; Subramanian, S. Biochem. 1981, 20(11), 3096-3102.
CrossRef - Gear, R.; Miaskowski, C.; Gordon, N.; Paul, S.; Heller, P.; Levine, J. Nature Med. 1996, 2(11), 1248.
CrossRef - Lagunin, A.; Gloriozova, T.; Dmitriev, A.; Volgina, N.; Poroikov, V. Bull. Exper. Biol. Med. 2013, 154(4), 521-524.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









