Potassium Dihydrogen Phosphate Purification Process by Crystallisation in the Presence of Chelating Agents
Ilia Komendo , Anna Amelina and Anna Zharova
, Anna Amelina and Anna Zharova
NRC «Kurchatov Institute» – IREA, Moscow, Russia.
Corresponding Author E-mail: i.comendo@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340602
Article Received on : 02-11-2018
Article Accepted on : 03-12-2018
Article Published : 05 Dec 2018
This article describes the investigation of the purification process of potassium dihydrogen phosphate (KH2PO4, KDP) from iron and chromium impurities by crystallisation in the presence of different chelating agents – chelators. The best conditions of purification process such as a chelator type and its amount, reaction time were determined by experiments. The most effective chelating agents were selected. The requirements for raw material for the purification process were defined.
KEYWORDS:Chelating Agents; Chelators; Inorganic Salts Purification; Potassium Dihydrogen Phosphate
Download this article as:| Copy the following to cite this article: Komendo I, Amelina A, Zharova A. Potassium Dihydrogen Phosphate Purification Process by Crystallisation in the Presence of Chelating Agents. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Komendo I, Amelina A, Zharova A. Potassium Dihydrogen Phosphate Purification Process by Crystallisation in the Presence of Chelating Agents. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=53447 |
Introduction
High-purity potassium dihydrogen phosphate (KDP) is widely used as a raw material for single crystal growth. KDP single crystals are applied in different areas of implementation. The large ones (40 cm in height) are used as frequency doublers, and Q-switches in high-beam facilities such as National Ignition Facility in the USA,1,2 and the smaller ones are usually taking the application in fast neutron detectors systems.3,4 For single crystal growth, it is necessary to use a raw material with low concentrations of metal impurities especially iron and chromium due to its strong influence on the crystal growth rate and crystalline perfection.5,6 The requirements for the single crystal growth raw material are <50 ppb (parts per billion) of iron and chromium impurities per each. Iron is one of the most common impurities. As also iron and chromium are the widespread components of chemical industry stainless steels (AISI 321 or 304, for example). It is well known, that mentioned impurities are concentrating in solid phase during crystallization,7,8 therefore designing of a method to prevent cocrystallisation of iron and chromium in industrial processes of obtaining high-purity KDP is an actual problem.
The effective method of obtaining inorganic salts purified from impurities of d-metals is to use chelating agents (chelators) during crystallization.9 D-metals tend to form strong chelate complexes due to its electronic shell structure, but phosphate ligands of KDP can form complexes with metal ions too. Hence, competitive complexes formation will occur, and it is not clear how efficient is to use chelating agents in such strong complexing media as potassium dihydrogen phosphate solutions. Present work aims to choose the most efficient chelating agent and conditions which will allow obtaining potassium dihydrogen phosphate purified from iron and chromium impurities.
Experimental
Apparatus and Materials
The following reagents were used: potassium dihydrogen phosphate, chromium(III) and iron(III) chlorides, chelating agents DTPA (diethylenetriaminepentaacetic acid), NTP (nitrilotris(methylene))triphosphonic acid), NTA (2,2′,2”-nitrilotriacetic acid), EHDP (1-hydroxy-1-phosphonoethylphosphonic acid), EDTA (ethylenediaminetetraacetic acid). Deionised water with its specific resistivity of ≥18.0 МOhм was used to prepare solutions.
Experimental Procedure
Crystallisation experiments were performed by cooling to a room temperature KDP solution saturated at 80°С. During crystallisation, continuous stirring on a magnetic stirrer was sustained. For maintaining of solutions on desired temperature water bath was used. The optical absorbance of coloured complexes was measured on a photoelectric colourimeter in optical glass cells with a length of 50 mm.
Purification efficiency was estimated based on the purification coefficient (Kp) value which was calculated as a ratio of the initial concentration of the impurity to its concentration after purification
![]()
Distribution coefficient (Kd) was calculated as a ratio of an impurity concentration in crystals to its concentration in mother liquor:
![]()
Results and Discussion
At the baseline, optimal conditions of chelating agents applying were determined. It is well known that the reaction between chromium ions and EDTA proceeds slowly.10 In potassium dihydrogen phosphate solutions reaction rate might be lower because of competitive complexing between EDTA and phosphate ligands. It is important to note, that Cr-EDTA is a convenient system to determine the kinetics of complexing by photocolorimetric technique due to the Cr-EDTA complex have intense violet colour. To determine time sufficient to complete the formation of complex, kinetics of complexing was measured. 15 ppm of Cr(III) was added to the 100 ml of saturated at 80°C KDP solution and maintained at 90°C for 3 hours to form chromium complex with phosphate ligands of KDP. Then 150 ppm of EDTA was added to the solution and maintained at 90°C. Kinetics of formation of Cr-EDTA complex was determined by the colourimetric technique using the following sequence of operations: 10 ml aliquot of KDP solution was made to volume 25 ml and optical absorbance was measured at the wavelength of 543 nm.10 As it can be seen (Fig. 1.), at the temperature of 90°C the sufficient time to form Cr-EDTA chelate in concentrated KDP solution equals 2.5 hours. At the boiling temperature, the maximum absorbance of Cr-EDTA complex was reached in 1 hour.
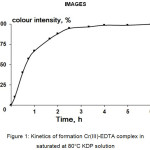 |
Figure 1: Kinetics of formation Cr(III)-EDTA complex in saturated at 80°C KDP solution.Click here to View figure |
Next step of the investigation was to find the optimal amount of chelating agent to purify KDP from iron and chromium impurities. Influence of the added amount of EDTA on the purification coefficient was investigated. Studied systems were Fe-EDTA and Cr-EDTA. Purification of KDP with the initial concentration of the controlled impurity of 5 ppm was performed by coolingcrystallisation of the saturated at 80°C solution. Then crystalline product was separated from the mother liquor by filtration and washed by deionized water. Iron and chromium concentrations were measured by the photocolorimetric technique described.11 According to the Figures 2A and 2B, the purification coefficient Kp reaches a plateau when EDTA was added of 0.35% mass (0.17% molar).
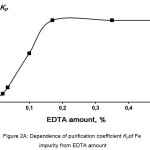 |
Figure 2A: Dependence of purification coefficient Kp of Fe impurity from EDTA amount. |
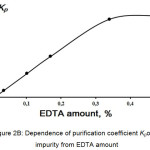 |
Figure 2B: Dependence of purification coefficient Kp of Cr impurity from EDTA amount.Click here to View figure |
Washing a crystalline product might be crucial when obtaining a fine-crystalline precipitate. Mother liquor wetting and covering crystals with the high surface area can be the source of contamination.The necessity of executing the procedure of washing a product was estimated. The cooling crystallisation of saturated at 80°C KDP solution was performed with the addition of 0.35% EDTA. The crystalline product was separated from the mother liquor by filtration and concentrations of iron and chromium impurities in crystals were measured, then crystals were once washed by deionized water, and iron and chromium concentrations were measured again. The efficiency of such procedure was estimated based on values of purification coefficients. Statistical sampling of experiments performed presented in Table 1. Operation of washing of crystals allows to raising the purification coefficients at the average in 3 times.
Table 1: Kp values of Fe and Cr impurities before and after washing KDP crystals.
|
Impurity |
Experiment №1 |
Experiment №2 |
Experiment №3 |
Experiment №4 |
||||
|
Purification coefficient (Kp) |
||||||||
|
n/w |
w |
n/w |
w |
n/w |
w |
n/w |
w |
|
|
Fe |
10 |
30 |
10 |
27 |
6 |
13 |
7 |
20 |
|
Cr |
8 |
25 |
9 |
30 |
3 |
9 |
3 |
8 |
w – crystalline product was once washed by deionized water.
n/w – crystalline product was not washed.
Distribution coefficients of iron and chromium impurities were determined. To the saturated at 80°C KDP solution 0.35% of following chelating agents: NTA, EDTA, DTPA, NTP, EHDP were added. The solution was maintained at the boiling temperature for 1 hour, then the amount of evaporated water was compensated, and the solution was cooled down to the room temperature with continuous stirring. Crystals were separated from the mother liquor and once washed by deionized water. Concentrations of iron and chromium were measured in crystalline product and mother liquor.
Comparison of distribution coefficients values determined for crystallisation in the presence of chelating agent with those determined for common coolingcrystallisationallows suggesting that the most stable complexes with Fe and Cr ions form carboxylated amineagents such as NTA, EDTA, DTPA. Phosphorous-containing chelators presumably not form complexes with Fe and Cr ions in concentrated potassium dihydrogen phosphate solutions. However, KDP crystallisation in the presence of NTA and homologues allows reducing distribution coefficients by several orders of magnitude.
Table 2: Cr and Fe distribution coefficients values comparison.
|
Impurity |
Crystallisation without chelating agent8 |
Chelating agent |
||||
|
NTA |
EDTA |
DTPA |
EHDP |
NTP |
||
|
Distribution coefficient (Kd) |
||||||
|
Cr |
24.3 |
0.6 |
0.02 |
0.06 |
1.0 |
1.6 |
|
Fe |
2.74 |
0.1 |
0.005 |
0.005 |
0.73 |
1.8 |
Finally, to set the requirements to a raw material for high-purity potassium dihydrogen phosphate producing, the dependence of the purification efficiency against initial impurity concentration was established. According to Fig. 3 initial concentration of impurity should not overdraw 1.5-2 ppm.
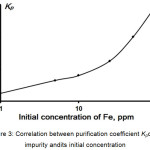 |
Figure 3: Correlation between purification coefficient Kpof Fe impurity andits initial concentration. |
It is possible to raise the purification coefficient by adding more than 0.35 % of a chelating agent, but this way leads to raising the concentration of total organic carbon contributed with residual amounts of chelator in crystalline product. We controlled the residual concentration of EDTA in KDP by the photocolorimetric technique based on the addition of Co(II) ions to probe and further oxidation to the Co(III) by hydrogen peroxide. Once the Co(III)-EDTA complex was formed, its optical absorbance proportional the EDTA concentration was measured at the wavelength of 540 nm.12 The mentioned method allows determining EDTA concentration in KDP down to 5 ppm. Addition of chelator, EDTA for example, during crystallisation in 0.35% leads to the residual concentration of chelatorin crystalline product lower than 5 ppm.
Conclusions
Different chelators were tried in the purification process of potassium dihydrogen phosphate. It was found that addition of EDTA and DTPA during crystallisation allows reducing distribution coefficients of iron and chromium impurities by the 3 degrees of magnitude.
Reaction rate between chromium and EDTA and consequently other chelating agents in concentrated potassium dihydrogen phosphate is slow due to the competing complexation reactions between phosphate ligands of KDP and ligands of the chelator.
The optimal amount of chelating agent for reducing iron and chromium impurities concentrations in KDP down to <0.05 ppm equals 0.35% with its initial concentrations 1.5-2.0 ppm.
Washing of crystalline product is necessary. Such operation allows raising the purification coefficient almost 3 times.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
Current work is supported by the grant of Ministry of Education and Science of Russia 14.625.21.0039, project identifier RFMEFI62516X0039.
References
- Roy, U. N. IETE J. Res. 1997, 43 (2-3), 245-249.
CrossRef - Hawley-Fedder, R.; Robey, H.; Biesada, T.; DeHeaven, M.; Floyd, R.; Burnham, A. Proceedings SPIE. 2001, 4102, 152–161.
CrossRef - Voronov,A.P.;Babenko,G.N.;Puzikov,V.M.;Roshal,A.D.;Salo,V.I. Crystallogr. Rep. 2008, 53(4), 708-712.
CrossRef - Voronov, A.P.;Salo, V.I.;Puzikov, V.M.;Tkachenko, V.F.;Vydai, Y.T. Crystallogr. Rep. 2006,51(4), 696-701.
CrossRef - Rashkovich, L.; Kronsky, N.J. Cryst.Growth. 1997, 182, 434-441.
CrossRef - Ding, J.; Wang, S.; Xu, X.; Gu, Q.; Liu, W.; Sun, Y.; Liu, G.; Zhu, S. J. Cryst.Growth. 2011, 334(1), 153-158.
CrossRef - Efremova, E.P.; Sukhanova, A.I.; Kuznetsov, V.A. Inorg. Mater. 2004, 40(6), 636-640.
CrossRef - Komendo, I.Y.; Mikhlin, A.L.; Volkov, P.A.; Dossovitskiy, A.E.; Allakhverdov, G. R. Inorg. Mater. 2018, 54(2), 171-175.
CrossRef - Beneš, J. Collect. Czech. Chem. C. 1969,34(5), 1514-1522.
CrossRef - Hedrick, C. E. J. Chem. Educ, September. 1965, 42(9), 479-480.
- Dosovitsky, A. E.; Komendo, I.Y.; Mikhlin, A. L.; Retivov, V. M.; Bulatitsky, K. K.; Rodchenkov, V. I. Ind. Lab. (in russian: Zavodskaya Laboratoriya. Diagnostika materialov). 2017, 83, 13-17.
- Paraneeiswaran, A.; Shukla, S. K.; Sathyaseelan, V. S.; Rao, T. S. International Journal of Applied Science and Biotechnology.2015, 3(4), 584-587.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









