Photochemical Method of Depositing Silver Films on the Surface Cotton Fabrics
M. S. Sataev , Sh. T. Koshkarbayeva, P. A. Abdurazova, R. S. Abzhalov, U. B. Nazarbek and R. A. Issaeva
, Sh. T. Koshkarbayeva, P. A. Abdurazova, R. S. Abzhalov, U. B. Nazarbek and R. A. Issaeva
M. Auezov South Kazakhstan State University, Shymkent, Kazakhstan.
Corresponding Author E-mail: malik_1943@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/340610
Article Received on : 25-07-2018
Article Accepted on : 12-11-2018
Article Published : 16 Nov 2018
A technology has been developed for the production of silver films on cotton fabrics, which provides for the preliminary application film of monochloride of copper to the surface of the fabric. Physicochemical and photochemical processes are considered, leading to the formation chloride of monovalent copper, providing sufficient adhesion to the substrate. The subsequent treatment with nitrate of silver, when the substitution reaction proceeds, leads to the formation chloride of silver, which under the influence of sunlight is restored to elemental silver. It is shown that in this case also the oxidation of monovalent copper occurs to form soluble salts, easily removable by rinsing. Since the proposed technology does not require special equipment, and the chemical reagents used are not deficient, It can be used to apply bactericidal films of silver on various household products and medical use in conventional laundries or home conditions.
KEYWORDS:Adhesion; Chloride of Silver; Cotton Fabric; Monochloride of Copper; Photochemical Process; Silver
Download this article as:| Copy the following to cite this article: Sataev M. S, Koshkarbayeva S. T, Abdurazova P. A, Abzhalov R. S, Nazarbek U. B, Issaeva R. A. Photochemical Method of Depositing Silver Films on the Surface Cotton Fabrics. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Sataev M. S, Koshkarbayeva S. T, Abdurazova P. A, Abzhalov R. S, Nazarbek U. B, Issaeva R. A. Photochemical Method of Depositing Silver Films on the Surface Cotton Fabrics. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52682 |
Introduction
It is known that most of the univalent compounds of the copper subgroup under slight heating and under the action of light disintegrate easily.1 The sensitivity of silver halides is used for photographic emulsions. In this case, the photosensitivity is related to the semiconducting properties of monovalent metal halides of the copper subgroup. These properties of halides can be used to produce metal films on the surface of various products in order to give them some new functional properties. In this case, in the first place are due to the bactericidal properties of silver. Silver has a phenomenal bactericidal and antiviral activity. In work2 the analysis of bactericidal properties of surface films containing elements of a subgroup of copper (copper, silver, gold) as separately, and in various combinations. These studies confirmed the high antibacterial properties of silver.
Therefore, a wide dissemination has been developed on the application of silver films to medical materials: implants, systems for drug delivery, antibacterial coatings for biomedical devices and antimicrobial packaging.3-8
Such materials are also used in household products: curtains, napkins, bandages, bactericidal inserts in various appliances (refrigerators, fans, air conditioners), articles of clothing (socks, insoles, etc.).9,10
It should be noted that along with the high bactericidal properties of silver itself, Silver compounds are highly toxic substances, therefore bactericidal films should contain only elemental silver. In this case, electrically neutral metal particles are more evenly distributed in the matrix (film former), providing reliable protection against microorganisms.
In the literature, a number of methods for applying metallic films to nonmetallic materials are given, which can be used to film silver films on cotton materials. It should be taken into account that antibacterial films should not degrade the consumer properties of the woven product and, at the same time, ensure bactericidal activity after washing and wrinkling. In view of these requirements, the following methods are suitable for these purposes.
From physical methods, it is known to use magnetron sputtering in a vacuum chamber of metal particles (including silver), followed by deposition on the surface of the material.11 The method is based on the use of an anomalous glow discharge in an inert gas, at which positively charged ions, formed in the discharge, bombard the surface of the cathode in the erosion zone and knock out metal particles, which are then deposited as a thin layer on the surface of the material being processed. The application of the metal coating is carried out without the use of chemical substances, polluting the environment. In addition, the high kinetic energy of particles leaving the surface of the cathode, provides a good level of adhesion of the resulting film to the substrate. Disadvantage: the use of special expensive equipment.
In some physical methods of silvering, it is proposed to obtain silver nanoparticles, which are then applied by gas-phase, plasma or vacuum spraying.12,13 The disadvantage of these methods is the need for preliminary production of silver nanoparticles, difficulties of metallization of internal surfaces of porous materials, complexity of coating thickness regulation.
To physical it is possible to carry and a way of reception of medical armbands, in which silver nanoparticles precipitated as a result of a chemical reaction, physically dispersed between fibrous tissues.14 These products do not exhibit sufficient antimicrobial activity due to the low content of silver in them, have some problems with their structure, consisting in the fact that the size and shape of nanoparticles are not uniform, the distribution of particles is difficult to control, it is difficult to regulate the concentration and content of silver. In addition, since silver is not chemically bonded, but is physically attached to products, then the loss of finely dispersed nanoparticles of silver from these products is likely.
In the works15-17 for the preparation of silver films, preliminary films phosphide of copper were applied to the fabric materials, which were then transformed into silver. The possibility of using this method is shown, for both synthetic and natural tissues. The disadvantage is the use as a reducing agent of toxic phosphine and the consequent need for work in hermetic conditions.
To obtain bactericidal films of metal particles of the copper subgroup, photochemical methods are also used.
A method is known including photochemical reduction of transition metal compounds on the surface of microspheres of polymers, which act as centers of sorption of metal nanoparticles, provide the possibility of obtaining the latter in the form of stable dispersions. Photochemical synthesis of copper nanoparticles, silver and gold on the surface of microspheres of polymers.18,19 It is characterized by greater purity of the nanoparticles formed, since there are no by-products obtained by using chemical reducing agents. But from the given material the mechanism of photochemical reaction of formation of metal nanoparticles is unclear and the effect on this of the structure of the dielectric. The disadvantage of the method is also that, that a photochemical reaction with the formation of silver proceeds only with a certain structure of latexes, which greatly narrows the scope of the method.
In the work2 a combination of chemical and photochemical methods produced films containing elements of copper subgroup in various combinations. In order to obtain silver films, the fabric was treated in solutions containing suspensions chloride of silver, which is inconvenient both from a technological point of view, and also can not provide the necessary adhesion of the film to the substrate.
From these data it follows that the problem of creating a new technology for obtaining bactericidal films of silver is topical.
Materials and Methods
In this paper, it is proposed to use physicochemical processes for obtain silver films, flowing in thin layers of solutions of electrolytes under the influence of sunlight. For the photochemical reduction of silver, it is necessary to create a photosensitive layer of a semiconductor silver halide on the surface of the tissue. Using for this purpose a suspension of silver halides or their direct preparation by exchange reactions on the surface of the tissue does not ensure proper adhesion of the film to the tissue.
Therefore, the technological scheme was developed (Fig. 1), which provided for the preliminary production of a layer monochloride of copper by the photochemical method on the surface of the tissue, its transformation into chloride of silver and photochemical reduction to silver.
For laboratory studies of individual processes, cotton gauze fabric (art. AA010278) was used, widely used for medical purposes.
The structure and composition of the films at the individual stages of the processes were studied using a scanning electron microscope ISM-6490-LV (JEOL, Japan), allowing to obtain electronic images (photographs) of individual sections (spectra) of the surface for various given magnifications. At the same time, it is possible to obtain elemental spectra compositions in the form of a table.
Samples cut from the tissue were wetted in a solution of CuCl2 held over the solution until excess solution was removed and exposed to the sun until completely dry. At the same time, a black film was formed on the surface of the tissue, characteristic for fine particles of metals obtained by chemical reduction. This indicates that as a result of these processes there is a transformation CuCl2→ CuCl→ Cu (Fig. 1, position 1-8). The resulting layer of elemental copper is stable, while the chlorine of copper formed during the photochemical reaction is in the solid phase. When washing, the CuCl2 transition occurs in a soluble form, which contributes to the oxidation of elemental copper (Fig. 1, position 9.10) with the reverse formation of monovalent chlorides. Washing was carried out by dipping the sample three times in a vessel with distilled water, changing each time water. As a result of washing, the surface of the sample is purified from the excess amount chloride of copper that has not reacted. This also removes the particles monochloride of copper, poorly adhered to the surface of the tissue. Thus, after washing, only a film of copper chloride particles firmly bound to the base remains (Fig. 1, position 11).
In the further processing of this sample with a solution of silver nitrate, chloride of copper are replaced with chlorides of silver (Fig. 1, position 12-13).
If we take into account the relatively porous nature of the chlorides and the fact that the product of the solubility of copper (I) chloride is 1,02∙10-6, and chloride of silver, 1 ∙ 10-10, it follows that the formation chloride of silver occurs rapidly and practically quantitatively.
After this, the sample is again exposed to sunlight and when the specimen dries completely, on its surface a film of dark color is formed (Fig. 1, position 14). Washing of the film from water-soluble compounds is carried out first by dipping in a vessel with distilled water, then flowing tap water (Fig. 1, position 14-16). The film thus obtained consists of elemental silver (Fig. 1, position 17).
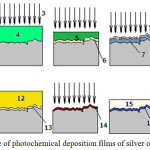 |
Figure 1: Scheme of photochemical deposition films of silver on cotton fabrics. |
1-cotton fabric; 2-solution of copper chloride; 3-rays of the sun; 4-increase in concentration due to evaporation of moisture in the sorption layer; 5-saturated solution of copper chloride; 6-layer monochloride of copper; 7-layer of elemental copper; 8-layer chloride of copper unreacted; 9-water; 10 – a layer of copper monochloride; 11-layer monochloride of copper after washing; 12- solution of silver nitrate; 13-layer of silver chloride; 14,16- layer of elemental silver after a photochemical reaction; 15- water; 17- a purified layer of silver.
Results and Discussion
Cotton fabrics by 97-98% consist of cellulose. Macromolecules in natural cellulose are combined into bundles, between which there are micropores. The bundle of macromolecules are connected in its turn to fibrils (the finest fibers), and bundles of fibrils into elementary fibers. Micro- and macropores are filled with air. Each elementary unit of the macromolecule of cellulose – anhydroglucose – contains three hydroxyls of alcoholic, which makes it very sensitive to the action of oxidants. As a result of oxidation of alcoholic hydroxyl groups in macromolecules of cellulose, new carbonyl and carboxyl functional groups. Oxidation starts from the surface of the fiber, and then gradually moves into deeper layers, at this the amorphous part is first oxidized, and then the crystalline sections.20
Dichloride of copper with simultaneous exposure to sunlight plays the role of an oxidizer and interacts with cellulose. The result of this interaction is the formation chloride of univalent copper according to the following reaction.
2СuCl2 + H2O+ R-OH =2 СuCl + 2 HCl+ R-OOH (1)
where R – is the individual hydroxyl group of the cellulose unit.
In preliminary experiments it was observed that the intensity of the black color of the copper film formed on the surface of the material by the photochemical method depends on the concentration of the initial CuCl2 solution. Therefore, considering that all copper contained in the CuCl2 solution passes into the film, the intensity of black at intermediate concentrations and by comparison, a change in the concentration of copper in the film was found during the course of the photochemical reaction. The quantitative characteristics of the intensity of the black films of the samples were determined using a computer. For this purpose, photographs of the investigated samples were entered into a separate file and using the editor “work with drawings” determined the change in the degree of brightness (in percent) when the dark color of the sample changes to white.
To study physicochemical processes, tissue samples were wetted with a solution CuCl2∙2H2O – 100 g/L. The amount of the solution retained by the sample was 2 × 5 cm was 0.6 ml. The content of copper in this layer of the solution is about 25∙10-3г. The experiments were carried out at a flux density of solar radiation of 200-220 W / m2 and an air temperature of 32°C.21
The transition of bivalent copper to monochlorine is explained as follows. Under the influence of sunlight, water is removed from the sorption film, which leads to an increase in the concentration of copper halide. This is evidenced by an increase in the intensity of the color of copper chloride (Figure 2 pos.1,2,3). An increase in the concentration of chlorides makes it possible for the reaction 1 to proceed with the formation monochloride of copper. The duration of this stage is about 30 minutes (Fig.3. position 1.2,3). Monochloride of copper is a binary semiconductor. Therefore, after the formation of the semiconductor layer, direct photochemical reduction of copper ions becomes possible, which also takes about 30 minutes, then there is a sharp slowing down of the process (Fig.3, position 4,5,6). The resulting particles of elemental copper, give the surface of the sample a dark color characteristic of thin films, obtained from solutions of salts with the help of chemical reducing agents (Figure 2, position 4,5,6).
In this case, the bulk of copper (80% of the copper chloride contained in the sorbed layer) is recovered without noticeable difficulties. At this stage, the rate of photochemical reaction is comparable to the rate of chemical or galvanic metallization. Further sharp slowing of the rate of photochemical reaction may occur for the following reasons: due to a decrease in the concentration of copper chloride; diffusion limitations of the processes associated with the oxidation of cellulose molecules; loss of electroconductive properties of solutions with their complete drying.
The formation of elemental copper occurs when exposed to a photon of sunlight.
![]()
Emerging vacancies (⊕) lead to the oxidation monochloride of copper.
![]()
Thus, the total photochemical reaction will have the form
2 CuCl →Cu + CuCl2 (4)
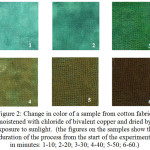 |
Figure 2: Change in color of a sample from cotton fabric moistened with chloride of bivalent copper and dried by exposure to sunlight. (the figures on the samples show the duration of the process from the start of the experiment in minutes: 1-10; 2-20; 3-30; 4-40; 5-50; 6-60. |
It is not possible to remove the dichloride of copper by washing, since its solutions (formed when dissolving solid crystals) immediately come into a reverse reaction with elemental copper, forming insoluble chloride of univalent copper.
Cu+CuCl2→2 CuCl (5)
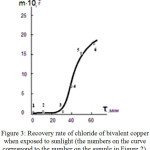 |
Figure 3: Recovery rate of chloride of bivalent copper when exposed to sunlight (the numbers on the curve correspond to the number on the sample in Figure 2). |
But in this case washing is useful, since in this case non-stoichiometric amounts of CuCl2 are removed, and poorly bound to the surface of the tissue particles of monochloride copper.
As a result of all these processes, the surface layer of the fabric will contain only a single-chloride copper that is well adherent to the base. In this case, the fabric acquires a uniform, monophonic light-green color, which indicates the formation of associates chloride of copper with macromolecules of cellulose. The formation of these associates is possible and is associated with high adhesion of the films.
The last impregnation of the tissue with a solution of silver nitrate leads to the course of the exchange reaction involving chloride of copper and the formation of a photosensitive layer chloride of silver on which, under the action of a solar photon,
![]()
and under the action of a vacancy, the oxidation reaction
![]()
Hence the total reaction will have the form
AgCl + CuCl → Ag + CuCl2 (8)
This does not exclude the oxidation of the deep molecules of the alcohol groups of cellulose, which were not oxidized by chloride of copper (according to reaction 1), but can be oxidized by chloride of silver.
In this case, because of the more positive silver potential, there is no interaction between Ag and CuCl2, after washing, only a layer of elemental silver remains on the surface of the fabric. This is confirmed by the data obtained by means of an electron microscope (Fig. 4.5). Figure 4 shows the electronic image and the elemental composition of the original sample from cotton fabric. The image of this sample after all the operations on applying silver films confirm the formation of a silver film as a result of the physicochemical and photochemical processes (Fig. 5). As can be seen from the data of the elemental composition, the silver content in the surface layer is 13.59%. In this case, the absence in the copper layer indicates that the complete substitution of copper chloride with silver chloride has passed. At the same time, the presence of a small amount of chlorine indicates an incomplete reduction chlorides of silver when exposed to sun rays. The reason for this is probably a partial screening of the chlorides.
Concentrations of solutions used for impregnating tissues depend on the type of tissue and the content of cellulose and other polymeric constituents in it. For this tissue, the concentration of CuCl2 was 30 g / L. This is the minimum concentration, which ensures the maximum darkening of the surface of white tissue during photochemical treatment. To obtain the AgCl film, a 2% AgNO3 solution was used, and a volume of solution was taken that provided a complete replacement of copper with silver and the formation of a film containing only elemental silver.
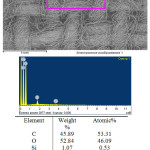 |
Figure 4: Electron picture and elemental composition of origin sample of the cotton textile. |
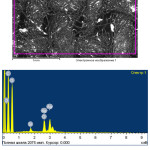 |
Figure 5: Electron picture and elemental composition of sample of the cotton textile treated in a solution of CuCl2 after photochemical deposition of a silver film (light sections of a silver particle). |
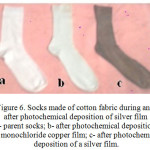 |
Figure 6: Socks made of cotton fabric during and after photochemical deposition of silver film a- parent socks; b- after photochemical deposition of monochloride copper film; c- after photochemical deposition of a silver film. |
In this case, the silver particles are evenly distributed over the surface of the tissue. Some inhomogeneity in particle size seems to be due to the difference in the energy characteristics of the tissue surface. The film has a high adhesion to the surface of the fabric and is well resistant to numerous creases and washings. So, with a 40-time deminishment, dark powder was not observed, and after washing 10 times in a solution containing a synthetic detergent powder, the silver content did not decrease.
Conclusion
Thus, physicochemical and photochemical processes that occur under the influence of solar rays lead to the creation of a layer of associated particles of monochloride copper with cellulose molecules, which ensures high adhesion of photochemical films of silver.
The use of photochemical technology makes it possible to obtain silver films on the surface of cotton fabrics in the absence of chemical reducers. The merits of the proposed method also include that it does not require special equipment, and the chemical reagents used are not deficient. Therefore, the technology based on the photochemical method can be used to apply bactericidal films to their own medical devices of various hospitals, even small launches. It is possible to apply films to large-size products: sheets, pillow cases, bathrobes, etc. Do not exclude the use of the method and at home, so in recent years, advertised socks with applied silver films.22 It is noted that in these socks legs are not preet. Figure 6 shows photos of cotton socks at various stages of photochemical deposition of silver films in a chemical laboratory.
References
- Grebenkina V.M.; Kartuzhanskii А.L.; Kostenko М.I.; Plachenov B.Т.; Rimland Е.Y.; Studdzinskii О.P. Jour. prikl. chem. 1990, 5, 1074-1080.
- Abdurazova, P.A.; Nazarbek, U.B.; Bolysbek, A.A.; Sarypbekova, N.K.; Kenzhibayeva, G.S.; Kambarova, G.A.; Sataev, M.S.; Koshkarbaeva, Sh.T.; Tleuova, A.B,; Perni, S.; Prokopovich, P. Colloids Surf. A Physicochem. Eng. Asp. 2017,532, 63-69.
- Lansdown, A. B. J Wound Care. 2002.11.125–130.
- Garas’ko, Ye.V.; Shilyayev, R.R.; Chulovskaya, S.A.; Parfenyuk, V.I. Vestn. Ivanovskoy meditsinskoy akademii. 2008, 3-4, 30-34.
- Klasen, H. J. I. Early uses. J Burns. 2000, 26, 117–130.
- White, R. J. Br J Nurs. 2001, 10 (15 Suppl.2), 3–8.
- Thomas, S.; McCubbin, P. J Wound Care. 2003, 12, 101–107.
- Darouiche, R. O. Clin Infect Dis. 1999, 29, 1371–1377.
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Amold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D; Winship, P.D.; Reid, H.I. J Antimicrob Chemother. 2004, 54, 1019–1024.
CrossRef - Korobov, Yu. S.; Filippov, M.A .; Karabanalov, M.S.; Legchilo, V.V. Phyzika i chimya obrabotki materialov. 2012, 2, 70-74.
- Kalita, V. I.; Komlev, D. I. Phyzika i chimya obrabotki materialov. 2008, 2, 48-51.
- Shkundina S. Novye protsessy i materialy v proizvodstve pechatnykh plat. Tekhnologii v elektronnoy promyshlennosti. 2009, 4,1-7.
- Tausarova, B.R.; Kutzhanova, A.Zh.; Suleimenova, M.Sh.; Chemical journal of Kazakhstan. 2016, 1(53), 116–129.
- Sataev, M.S.; Koshkarbaeva, Sh.T.; Perni, S.; Nauryzova, S.Z.; Prokopovich, P. Colloids Surf A Physicochem Eng Asp, 2015, 480, 384–389.
CrossRef - Sataev, M.S.; Koshkarbaeva, S.T.; Tleuova, A.B.; Perni, S.; Aidarova, S.B.; Prokopovich, Р. Colloids Surf A Physicochem Eng Asp. 2014, 442, 146–151.
CrossRef - Sataev, M.S.; Koshkarbaeva, Sh.T.; Tasboltaeva, A.B. Izvestiya vuzov. Tekhnologiya tekstilnoy promyshlennosti. 2013, 6 (348), 102-104.
- Boytsova, T.B.; Volkova, Ye.I. Sorosovskiy zhurnal. 2005, 9, 1, 1-6.
- Isayeva, Ye.I.; Gorbunova, V.V.; Boytsova, T.B.; Shchukarev, A.V.; Sirotinkin, N.V. Zhurnal obshchey khimii. 2006. 76. 5. 723-729.
- Fayzrakhmanova, G.M.; Bashkirov, V.N.; Grachev, A.N.; Zabelkin, S.А. Vestnik tekhnologicheskogo universiteta. 2012, 15, 101-103.
- Bubenchikov, A.A.; Nurakhmet, Y.Y.; Molodikh, V.O.; Rudenok, A. I. International Research Journal. 2016, 5,47, 59-62.
- Margaret Ip,; Sau Lai Lui; Vincent, K.; M. Poon.; Ivan Lung; Andrew Burd. Journal of medical microbiology. 2006, 55, 59-63.
CrossRef - Savadyan, E.Sh. Zhurnal Antibiotiki i chemotherapii. 1989, 11, 874-878.

This work is licensed under a Creative Commons Attribution 4.0 International License.









