Novel Green Synthesis of Graphene Layers using Zante Currants and Graphene Oxide
Mohd Zaid Ansari1 , Mohammad Nadeem Lone2, Shabana Sajid3 and Weqar Ahmad Siddiqui1
, Mohammad Nadeem Lone2, Shabana Sajid3 and Weqar Ahmad Siddiqui1
1Department of Applied Science and Humanities, Jamia Millia Islamia, New Delhi-110025, India.
2Department of Chemistry, Jamia Millia Islamia, New Delhi-110025, India.
3Department of Chemistry, G.F College, Rohilkhand University U.P –242001, India
Corresponding Author E-mail: wsiddiqui@jmi.ac.in
DOI : http://dx.doi.org/10.13005/ojc/340621
Article Received on : 09-10-2018
Article Accepted on : 05-11-2018
Article Published : 08 Nov 2018
The present work shows a facile route for the preparation of graphene layers and for the first time Zante currants extract used for the effective deoxygenation of graphene oxide has been reported. Zante currants (ZC) extract reduce effectively GO into few layered structures of graphene (FLG). The morphology of few layers graphene and graphene oxide (GO) were investigated by SEM and TEM. Reduction effect on graphene oxide confirm by other technique like Raman, FTIR, XRD and UV spectrophotometry. This procedure keep away the use of hazardous chemicals, thus providing a new hope for large scale production of chemically reduced graphene.
KEYWORDS:Few Layers Graphene (FLG) and Green Synthesis; Graphene Oxide (GO); Zante Currants (ZC)
Download this article as:| Copy the following to cite this article: Ansari M. Z, Lone M. N, Sajid S, Siddiqui W. A. Novel Green Synthesis of Graphene Layers using Zante Currants and Graphene Oxide. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Ansari M. Z, Lone M. N, Sajid S, Siddiqui W. A. Novel Green Synthesis of Graphene Layers using Zante Currants and Graphene Oxide. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52273 |
Introduction
Graphene has attracted a great attention of researchers in the current era of emerging noanochemistry field due to its optical, electronic, thermal, and mechanical properties.1 Among several methods “chemical reduction method” is one of the well-known methods that has been used extensively for the reduction of graphene oxide (GO) to few layer graphene (FLG). Generally, it is performed by applying some reducing agents like hydrazine hydrate, hydrogen-bromide, p-toluene sulphonic acid, and sodium borohydride.2-4 There is no doubt it is the simplest process but, this procedure has few drawbacks like, toxicity nature of reducing agents as well as its cost.,5 Sometimes formation of agglomeration of graphene layers limit the implementation of this procedure. To avoid all these drawbacks, many eco-friendly pathway and bio-reduction procedures are being adopted , where microorganisms or plants extracts perform as deoxygenating agents.6,7 Therefore, it is more beneficially to reduce GO by using natural based reductants.8 There are several studies in which natural based reducing agents Ficus-carica,9 vitamin C10,11 Gallic acid,12 tea solution,13,14 bacteria15 and alanine16 have been utilized for this purpose. However, the graphene oxide reacted by the above-dicussed natural reductants without support of external stabilizer generally shows agglomerated graphene layers, so the applications of these green procedure have not been completely satisfactory. Thus, extensive investigation is still required to design, cost effective green reducing agent, environmental-friendly, to produce graphene on large-scale.
Keeping in view, of all the above discussed issues and agents of reduction of graphene oxide, we have chosen a natural based reducing agent: Black raisins which is scientifically known as Zante currants. Zante currants is actually a good source carbohydrate, containing mainly reducing sugars like glucose and fructose,17,18 anthocyanins19 as well as large no of polyphenolic compound.20 These functionalities have enough potential to reduce graphene oxide.8,21,22 Out of these reducing sugars may be responsible for reduction of GO due to higher percentage present in Zante currant.21 Reducing sugar itself and their oxidized outcomes are environmentally friendly. Latter also act as a capping reagent in stabilizing few layers graphene in aqueous suspension.
In this short piece of work, we investigated the deoxygenating ability of Zante currant for the reduction of GO through green method. The morphology and structure determination of GO and FLG were characterized by XRD, SEM, TEM and XRD, FT-IR, UV-Vis, spectroscopy respectively.
Materials and Chemicals
Chemicals and solvents were of analytical grade. Graphite powder, hydrogen peroxide, potassium permanganate, sulphuric acid, nitric acid and ethanol were purchased from Sigma Aldrich. Zante currants and cellulose membrane were purchased from the market. All the solutions were made using de-ionized (DI) water.
Synthesis
Synthesis of Zante Currants Extract
Initially 200 g of ZC were saturated in 300 mL deionized water for 30 min at 95 °C. Then, the extracted solution of ZC was sieved through cellulose membrane (0.45 μm) under suitable conditions to remove impurity.
Synthesis of GO
Synthesis of GO was developed by using well known method (Hummers method). In this method, initially a mixture of HNO3 (5 mL) and graphite flakes (2.0 g) was prepared in which conc. H2SO4 (70 mL) was added and the solution was left for cooling in ice bath. After cooling, six equal portions of KMnO4 (2 g at every 15 min) was added slowly at the temperature of less than 5ºC. Resulting mixture was put on stirring for 60 min in a water bath at 35°C and very slowly 100 mL of water was added that increase the temperature up to 100°C. Outside heating was implicated only up to 10 min to maintain the temperature 100°C, and the solution was cooled in a water bath. Furthermore, after gap of 20 min. 300 mL of water was poured to produce another exotherm. Finally, after 24 hr 3 mL of 30% H2O2 was added at room temperature and the resulting mixture was imperilled to centrifugation, multiple washing and vacuum drying that resulted into 900 mg of solid GO.
Reduction of graphene oxide by Zante currants Extract
100 mL of deionized water was added to 100 mg of GO to make aqueous suspension and sonicate the solution at least for 1 hr. Then 100 mL of prepared ZC extract and 10 mL of ammonia solution was added slowly into the solution and kept the mixture in a thermostatic bath at 95°C for 48 hr that resulted in dark black suspension comes out which confirms the formation of few layer graphene. Then, multiple washing was done with deionized water and three times by ether respectively, to eliminate any impurities of FLG.
Characterization of the Prepared Samples
Perkin Elmer FT-IR Spectrometer was used for recoding the IR spectra of GO and FLG on KBr pellets and values are given in cm-1, with the aid of X-ray diffraction (Rigaku Corporation, Tokyo, Japan) operating at 40 kV and a current of 30 mA with Cu Kα radiation (λ = 1.54 Å) with the scan rate of 4 degree/min the crystal structure of the GO and FLG were investigated.24 Perkin Elmer Lambda 35 (Perkin Elmer Life and Analytical Sciences, CT, USA) in the wavelength range of A200-800 nm was used for recording the UV-Vis. spectra of GO and FLG.
While Raman spectra was determined by LabRAM (HR800, JY). Morphological structure was determined by Field Emission Scanning Microscope (SIGMA VP) Zeiss, Germany and HR-TEM (F30 S-Twin) Technai.
Results and Discussion
X-ray Diffraction Analysis of Graphite GO and FLG
We characterized the graphite, GO and FLG by XRD for the determination of dispersion of graphite sheets and reduction of GO into FLG. As shown in Fig. 1 the diffraction peaks of graphite and GO were found at 2θ = 26.5º and 2θ = 11.3º,23,24 however, after the GO was reduced to graphene nanosheets, the diffraction peak of FLG appeared at 2θ=22.3º.25 (Fig.1). Furthermore, in case of GO the d-gaping of 0.79 nm was observed which is more in comparison to pristine graphite d-spacing (0.34 nm), due to addition of oxygen functionalities in graphite layers . These results demonstrate that initially graphite was in compact form and then GO sheets were individually dispersed and this dispersion was vanished after reduction as the peak positioned at 11.3° vanished, confirmed the reduction of GO with d-spacing 0.38 nm. This clearly shows the removal of oxygen functionalities from GO, lead honeycomb network which confirm the formation of graphene nanosheets.25,26
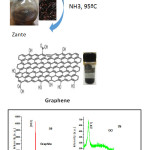 |
Figure 1: The XRD patterns of graphite, GO and FLG. |
Raman Analysis
The Raman spectrum of GO shows two bands G and D at 1605 cm– 1 and 1353 cm-1 which arises due to E2g phonon of the sp2 C atoms and the breathing mode of k point phonons of A1g symmetry, respectively (Fig. 2). In case of FLG, the G band was slightly shifted from the position of GO and observed at 1600 cm-1 (Fig. 2). While the D band shows the sign of disorder, which appeared due to few defects like grain boundaries, vacancies and amorphous carbon species.27,28 In addition, the intensity proportion of the D and G bands indicates the quality of the product as the band intensity ratio, ID/IG, increased from 0.86 (GO) to 0.96 (FLG). These changes are well supported by the available literature.29,30 Two bands were observed at 2688 cm-1 and 2900 cm-1. First band called as 2D band appears at 2688 cm-1, which tells about of graphene layers number. Here, the broad band observed, which confirms that the prepared FLG contains some defects. D–G peak combination create a second order peak called as S3 band appears at (2900 cm-1).31 The increased in ID/IG ratio and complete development of 2D and S3 band in FLG confirms better graphitization.31
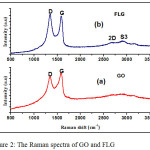 |
Figure 2: The Raman spectra of GO and FLG. |
FT-IR Analysis
The prepared GO and FLG were also analysed by FTIR spectroscopy revealed in Fig. 3. The FTIR spectrum of GO shows a strong absorption band at 1714 cm-1 due to the C=O stretching of the carbonyl group and other broad band at 3408 cm-1due to the O–H stretching mode. Besides, very strong bands at 1629 cm-1 due to the C=C stretching band and 1048 cm-1 due to the C–O stretching (epoxy or alkoxy) were observed.21 While in case of FLG, these characteristic absorption bands were missing or dramatically decreased which indicates the reduction of GO.21,32
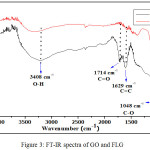 |
Figure 3: FT-IR spectra of GO and FLG. |
UV-Vis Spectrophotometry Analysis
The UV-Vis spectrum of GO and FLG are present in Fig. 4. Graphene oxide shows two absorption peaks appears at 233 and 368 nm. Peak at 233 nm refers to (π-π* transition of C=C bond) which transfer to 260 nm in FLG due to deoxygenation of graphene oxide as well as rebuilding of the conjugated aromatic structure.33,34 A bump appears at 320 nm in GO be accredited to n-π* transition of C=O bond.35 This bump dis-appeared when ZC reduces GO in spectrum of FLG.36 Deoxygenation process is also confirmed by change in colour of solution from dark brown to black.
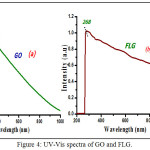 |
Figure 4: UV-Vis spectra of GO and FLG. |
Morphological Characterization
The morphology of GO and FLG were examined by FE-SEM and TEM with SAED techniques. The FE-SEM images obtained for GO and FLG are shown in Fig. 5(a), (b and c) separately. It can be clearly seen from Fig. 5(a) that GO sheets are exfoliated to each other and in case of Fig. 5(b), flower petals and sheet-like morphology with a micro meter size is clearly observed that confirms the reduction effect.36 The morphological characteristics of FLG were further investigated by TEM as well as HRTEM images with selected area electron diffraction (SAED) measurements in Fig. 6(a) (b), (c) and (d) respectively. The HR-TEM image of FLG demonstrates the formation of transparent layered graphene due to the reduction and SAED image proves a symmetric six-fold pattern with corresponding Miller-Bravais indices which refers to few layers’ grapheme.37
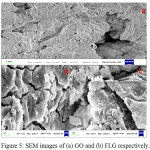 |
Figure 5: SEM images of (a) GO and (b) FLG respectively. |
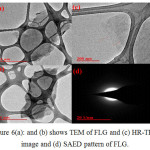 |
Figure 6(a): and (b) shows TEM of FLG and (c) HR-TEM image and (d) SAED pattern of FLG. Click here to View figure |
Conclusion
Finally, Zante currant considered as a natural reductant for the deoxygenation of graphene oxide. The GO and FLG were well characterized by some physical and chemical techniques. The irreplaceable advantages of ZC extract over chemical reduction method are environmentally friendly, facile synthesis of reduction, cost-effective, efficient reduction of GO. The reported procedure has outstanding reproducibility due to its simple procedure. Finally, FLG.
Acknowledgement
The authors acknowledge UGC, New Delhi for providing Non-Net fellowship to M. Z. Ansari.
References
- Whitener Jr, K. E., Sheehan, P. E., Graphene synthesis, Diamond Relat. Mater., 2014, 46, 25-34.
CrossRef - Stankovich, S., Dikin, D. A., Piner, R. D., Kohlhaas, K. A., Kleinhammes, A., Jia, Y., Ruoff, R. S., Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide, Carbon., 2007, 45(7), 1558-1565.
CrossRef - Ren, P. G., Yan, D. X., Ji, X., Chen, T., & Li, Z. M., Temperature dependence of graphene oxide reduced by hydrazine hydrate, Nanotechnology., 2010, 22(5), 055705
CrossRef - Shin, H. J., Kim, K. K., Benayad, A., Yoon, S. M., Park, H. K., Jung, I. S., Lee, Y. H., Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance, Adv.Func. Mater., 2009,19(12), 1987-1992.
CrossRef - Wang, Y., Zhang, P., Liu, C. F., Zhan, L., Li, Y. F., Huang, C. Z., Green and easy synthesis of biocompatible graphene for use as an anticoagulant, RSc. Adv., 2012, 2(6), 2322-2328.
- Mhamane, D., Ramadan, W., Fawzy, M., Rana, A., Dubey, M., Rode, C., Ogale, S., From graphite oxide to highly water dispersible functionalized graphene by single step plant extract-induced deoxygenation. Green Chem., 2011, 13(8), 1990-1996.
CrossRef - Nasrollahzadeh, M., Maham, M., Rostami-Vartooni, A., Bagherzadeh, M., & Sajadi, S. M., Barberry fruit extract assisted in situ green synthesis of Cu nanoparticles supported on a reduced graphene oxide–Fe 3 O 4 nanocomposite as a magnetically separable and reusable catalyst for the O-arylation of phenols with aryl halides under ligand-free conditions, RSc. Adv., 2015, 5(79), 64769-64780.
- Thakur, S., Karak, N., Alternative methods and nature-based reagents for the reduction of graphene oxide: A review, Carbon., 2015, 94, 224-242.
CrossRef - Ansari, M. Z., & Siddiqui, W. A., Deoxygenation of graphene oxide using biocompatible reducing agent Ficus carica (dried ripe fig), J of Nanostruct. Chemist., 2018 ,1-10.
CrossRef - Fernández-Merino, M. J., Guardia, L., Paredes, J. I., Villar-Rodil, S., Solís-Fernández, P., Martínez-Alonso, A., & Tascon, J. M. D., Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions, J.Phys.Chem. C.,2010, 114(14), 6426-6432.
- Zhang, J., Yang, H., Shen, G., Cheng, P., Zhang, J., & Guo, S., Reduction of graphene oxide via L-ascorbic acid. Chemi.Commun., 2010, 46(7), 1112-1114.
CrossRef - Li, J., Xiao, G., Chen, C., Li, R., & Yan, D., Superior dispersions of reduced graphene oxide synthesized by using gallic acid as a reductant and stabilizer, J.Mater.Chem.A., 2013, 1(4), 1481-1487.
CrossRef - Wang, Y., Shi, Z., & Yin, J., Facile synthesis of soluble graphene via a green reduction of graphene oxide in tea solution and its bio composites, ACS Appl. Mater. Interfaces., 2011 3(4), 1127-1133.
CrossRef - Akhavan, O., Kalaee, M., Alavi, Z. S., Ghiasi, S. M. A., & Esfandiar, A., Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide, Carbon., 2012, 50(8), 3015-3025.
CrossRef - Salas, E. C., Sun, Z., Lüttge, A., & Tour, J. M., Reduction of graphene oxide via bacterial respiration, ACS Nano., 2010, 4(8), 4852-4856.
- Wang, J., Salihi, E. C., & Šiller, L., Green reduction of graphene oxide using alanine, Mater. Sci. Eng., C., 2017, 72, 1-6.
CrossRef - Vasilopoulou, E., & Trichopoulou, A., Greek raisins: a traditional nutritious delicacy. Journal of Berry Research, 2014, 4(3), 117-125.
CrossRef - Nikolidaki, E. K., Chiou, A., Christea, M., Gkegka, A. P., Karvelas, M., & Karathanos, V. T., Sun dried Corinthian currant (Vitis Vinifera L., var. Apyrena) simple sugar profile and macronutrient characterization. Food Chem., 2017, 221, 365-372.
CrossRef - Chiou, A., Panagopoulou, E. A., Gatzali, F., De Marchi, S., & Karathanos, V. T., Anthocyanins content and antioxidant capacity of Corinthian currants (Vitis vinifera L., var. Apyrena). Food Chem., 2014, 146, 157-165.
CrossRef - Bennett, L. E., Jegasothy, H., Konczak, I., Frank, D., Sudharmarajan, S., & Clingeleffer, P. R., Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions, J. Funct. Foods., 2011, 3(2), 115-124.
CrossRef - Zhu, C., Guo, S., Fang, Y., & Dong, S., Reducing sugar: new functional molecules for the green synthesis of graphene nanosheets, ACS Nano., 2010, 4(4), 2429-2437.
CrossRef - Ansari, M. Z., Shoeb, M., Nayab, P. S., Mobin, M., Khan, I., & Siddiqi, W. A., Honey mediated green synthesis of graphene based NiO2/Cu2O nanocomposite (Gr@ NiO2/Cu2O NCs): Catalyst for the synthesis of functionalized Schiff-base derivatives, J. Alloys Compd., 2018, 738, 56-71.
CrossRef - Park, S., An, J., Potts, J. R., Velamakanni, A., Murali, S., & Ruoff, R. S., Hydrazine-reduction of graphite-and graphene oxide. Carbon, 2011, 49(9), 3019-3023.
CrossRef - Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., Tour, J. M. (). Improved synthesis of graphene oxide, ACS Nano., 2010, 4(8), 4806-4814.
CrossRef - Stobinski, L., Lesiak, B., Malolepszy, A., Mazurkiewicz, M., Mierzwa, B., Zemek, J., Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc.Relat. Phenom., 2014, 195, 145-154.
CrossRef - Johra, F. T., Lee, J. W., & Jung, W. G., Facile and safe graphene preparation on solution-based platform. J.Ind.Eng.Chem., 2014, 20(5), 2883-2887.
CrossRef - Ferrari, A. C., & Basko, D. M., Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat.Nanotech., 2013, 8(4), 235.
CrossRef - Cui, P., Lee, J., Hwang, E., & Lee, H., One-pot reduction of graphene oxide at sub-zero temperatures, Chem. Commun., 2011, 47(45), 12370-12372.
CrossRef - Fang, S., Huang, D., Lv, R., Bai, Y., Huang, Z. H., Gu, J., & Kang, F., Three-dimensional reduced graphene oxide powder for efficient microwave absorption in the S-band (2–4 GHz), RSc. Adv., 2017, 7(41), 25773-25779.
- Bajpai, R., Roy, S., Rafiee, J., Koratkar, N., & Misra, D. S., Graphene supported nickel nanoparticle as a viable replacement for platinum in dye sensitized solar cells, Nanoscale., 2012, 4(3), 926-930.
CrossRef - Moon, I. K., Lee, J., Ruoff, R. S., & Lee, H., Reduced graphene oxide by chemical graphitization., Nat. Commun., 2010, 1, 73.
CrossRef - Haghighi, B., & Tabrizi, M. A., Green-synthesis of reduced graphene oxide nanosheets using rose water and a survey on their characteristics and applications, RSC. Adv., 2013,3(32), 13365-13371.
- Akin, I., Zor, E., Bingol, H., & Ersoz, M.)., Green synthesis of reduced graphene oxide/polyaniline composite and its application for salt rejection by polysulfide-based composite membranes, J.Phys.Chem.B., 2014 118(21), 5707-5716.
CrossRef - Çiplak, Z., Yildiz, N., & Çalimli, A., Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods, Fullerenes, Nanotubes and Carbon Nanostructures., 2015, 23(4), 361-370.
CrossRef - Gurunathan, S., Han, J. W., & Kim, J. H., Green chemistry approach for the synthesis of biocompatible graphene, Int. J. Nanomed. 2013, 8, 2719.
CrossRef - Xu, C., Yuan, R. S., & Wang, X., Selective reduction of graphene oxide, New Carbon Mater., 2014, 29(1), 61-66.
CrossRef - Cui, T., Lv, R., Huang, Z. H., Zhu, H., Jia, Y., Chen, S., Kang, F., Low-temperature synthesis of multilayer graphene/amorphous carbon hybrid films and their potential application in solar cells. Nanoscale Res. Lett., 2012, 7(1), 453.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









