Characterization of Enhanced Antibacterial Effects of Silver Loaded Cerium Oxide Catalyst
Gusliani Eka Putri1 , Syukri Arief2, Novesar Jamarun2, Feni Rahayu Gusti1 and Annisa Novita Sary1
, Syukri Arief2, Novesar Jamarun2, Feni Rahayu Gusti1 and Annisa Novita Sary1
1Sekolah Tinggi Ilmu Kesehatan Syedza Saintika, Padang, West Sumatera, Indonesia.
2Department of Chemistry, Faculty of Mathematic and Natural Sciences, Universitas Andalas, Indonesia.
Corresponding Author E-mail: guslianiekaputri@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340629
Article Received on : 28-09-2018
Article Accepted on : 10-11-2018
Article Published : 15 Nov 2018
Silver-cerium nanoparticles had been successfully synthesized using the sol-gel method by silver nitrate as a source of silver and cerium nitrate hexahydrate as a source of cerium. The synthesized silver-cerium nanoparticles had been characterized by X-ray diffraction,transmission electron microscopy, and scanning electron microscopy-energy dispersive X-ray. Based on the results of XRD and TEM analysis showed silver-cerium nanoparticles were spherical with the dominant size range of 8.9 -12.73 nm. SEM-EDX analysis showed silver nanoparticles covered by cerium nanoparticles that were known as the core-shell structure. Silver nanoparticles doped with cerium nanoparticles (CeONP) showed an increase in inhibitory with an increase a zone of inhibition after being doped with cerium nanoparticles. The disinfection effect of Ag-doped CeONP was more pronounced on Staphylococcus aureus than Escherichia coli, although the difference was not wide.
KEYWORDS:Antimicrobacteial Activity; Cerium Nanoparticles; Silver Nanoparticles; Syhntesis
Download this article as:| Copy the following to cite this article: Putri G. E, Arief S, Jamarun N, Gusti F. R, Sary A. N. Characterization of Enhanced Antibacterial Effects of Silver Loaded Cerium Oxide Catalyst. Orient J Chem 2018;34(6). |
| Copy the following to cite this URL: Putri G. E, Arief S, Jamarun N, Gusti F. R, Sary A. N. Characterization of Enhanced Antibacterial Effects of Silver Loaded Cerium Oxide Catalyst. Orient J Chem 2018;34(6). Available from: http://www.orientjchem.org/?p=52615 |
Introduction
Cerium oxide was a material that has been widely researched lately because it has the ability to store oxygen so that it was possible to function as an oxygen storage in chemical catalytic processes.1,2 Cerium oxide buffer function can occur because cerium has two oxidation numbers namely trivalent Ce3+ and Ce4+ tetravalent. Therefore, cerium oxide acts as an oxygen provider under an oxygen-deprived environment. After the oxide surface gives oxygen, Ce4+ was reduced to Ce3+ because the extra electrons are left behind.3,4
When the oxide surface lacks oxygen, Ce3+ will tend to receive oxygen again. Cerium oxide nanoparticles have the ability to inactivate hydroxyl radicals of superoxide radicals, nitric oxide, and hydrogen peroxide.5,6 Another urgent problem of the health care system was the growing resistance that bacteria develop very quickly. Many ways were done to create organic antibacterial agents that can quickly kill bacteria. Among the antibacterial agents that are the current conversation was cerium oxide.7 Cerium oxide had a fast evolution rate. Cerium oxide provides a different mechanism for killing bacteria or limiting bacterial cell growth.8 Cerium oxide nanoparticles (CeONP) toxicity was associated with its properties as a reducing agent and killing bacteria by attacking membranes. Both mechanisms require direct contact with the cell wall and the spread of nanoparticles into bacteria so that it kills and weakens bacteria.7,9 So that the antibacterial properties of cerium oxide were better than doped with silver nanoparticles. In principle, the degradation of bacteria and viruses by silver ions was that silver particles will damage and penetrate the bacterial cell wall, then enter the bacterial thiol group and bind to sulfhydryl groups in bacteria so as to prevent the production of enzymes in bacteria. Furthermore, silver particles will inhibit DNA growth and eventually bacteria die.10 When applied as an antibacterial, it shown that CeONP was fed with silver not only releases silver ions but also increases the oxygen vacuum on the oxide surface. Thus, silver dopants introduce the Ag disinfection effect, which has been reported to be effective against several bacteria.11,12
In this reserach, we synthesized silver-cerium doped particles with nanometer size with a cost-effective sol-gel method and produced good quality particles. The resulting nanoparticles were used to inhibit the growth of Escherichia coli and Staphylococcus aureus bacteria.
Experimental
Chemicals and Reagents
Cerium (III) nitrate hexahydrate (Ce(NO3)3.6H2O) used for a precursor of CeO2, acetic acid, ammonia solution, 2-propanol, silver nitrate, polyvinyl alcohol (PVA), glucose was from Merck.
Synthesis of Silver-Cerium Nanoparticles
The source of the Ce metal was Ce(NO3)3.6H2O (cerium nitrate hexahydrate). Synthesis of cerium oxide (ceria) was done by making two solutions. The first solution was made by cerium nitrate hexahydrate dissolved in a mixture of a solvent of water and isopropanol with a ratio of 1:6. The second solution was synthesized by silver nitrate dissolved in 100 mL of water while being distilled until silver nitrate dissolved and ammonium hydroxide was added. The silver ion solution that formed was put into a glucose solution in a ratio of 18. In the solution, PVA was added with a variation of 3% concentration. After that, it was heated at a temperature of 60°C in an Erlenmeyer flask. During the heating process, the mixture is stirred using a magnetic stirrer until it is brown. The brown colloidal silver solution is put into the first solution a little bit and added ammonium hydroxide to a pH of 9 while in the stirrer. Then, it was refluxed for 30 minutes after it was filtered and will get a yellow precipitate. It was washed with isopropanol then dried for 5 hours at a temperature of 60°C so that yellow powder was obtained. It was calcined at 500°C for 2 hours and silver-cerium particles were obtained.
Results and Discussion
XRD Analysis
Figure 1 shown the XRD pattern of nanomaterials produced. The appearance of similar XRD peaks has been observed in JCPDS no. 00-041-1402 data with a hexagonal crystal structure and crystal size ranging from 18.61-30.01 nm for silver nanoparticles. Cerium oxide nanomaterials adjusted to JCPDS data No. 01-073-6318 with a cubic crystal structure with a size of crystal 11.94-27.97 nm. After Ag nanoparticles were doped with cerium oxide a histogram curve was produced which consisted of Ag and Ce peaks. The crystal size of the silver-cerium nanoparticles produced was calculated using the Debye Scherrer equation with the wavelength value, 2Ɵ and FWHM resulting from the XRD test.13 From the calculation results obtained the size of crystals 8.9 -12.73 nm. The presence of Ag−O bonds at the interface between the nanoparticles and the supporting oxide (CeO2) was also detected. The Ag−O interatomic distance decreases with decreasing nanoparticle size.14 Based on the analysis, silver, cerium and silver-cerium particles with a small size of 100 nm were indeed produced with the nano-sized material. Based on Fig. 1C, it can be shown that several peaks coincide because they are in the same area in position of 2Ɵ. The complexity of cerium oxide nanoparticles XRD spectrum is probably due to the presence of crystalline organic metallic complex like at 58,56 ° 2Ɵ (Fig. 1 A). This smaller crystallite morphology plays important role in biological applications of nanostructures.15
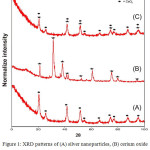 |
Figure 1: XRD patterns of (A) silver nanoparticles, (B) cerium oxide nanoparticles and (C) silver- cerium nanoparticles. |
TEM Analysis
The results of the TEM images of nanoparticles can be shown in Figure 2. Fig. 2A and 2B were TEM images of silver nanoparticles and cerium oxide nanoparticles. They were spherical with uniform size and shape. The measurement results with XRD characterization by using the Debye Scherrer equation to calculate the crystal size. These results were confirmed and strengthened by calculating the particle size of the material produced using image J software image. The results of shown that size of silver cerium nanoparticles ranging from 8.8 to 12.93 nm. Kayama, et al.,16 and Sing, et al.,17 also reported produced silver-cerium nanoparticles were spherical.
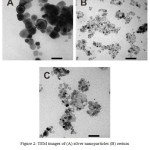 |
Figure 2: TEM images of (A) silver nanoparticles (B) cerium oxide nanoparticles and (C) silver-cerium nanoparticles. |
SEM-EDX
SEM-EDX shown morphological analysis of the material produced. Figure 3A was an Ag nanoparticle with a uniform particle size. Figure 3B was a uniform size cerium oxide nanoparticle scattered throughout the surface. Figure 3C was bimetallic of silver-cerium that was assumed Ag nanoparticles covered by cerium nanoparticles on the surface known as core-shell structured nanoparticles (Fig. 3D). Hybrid metal nanoparticles with core-shell structures have advantages such as unique optical properties, high magnetic properties, high electronic properties, and good catalytic properties that monometallic do not have.18
 |
Figure 3: SEM images of (A) silver nanoparticles (B) cerium oxide nanoparticles (C) silver-cerium nanoparticles and (D) core-shell structures of silver-cerium nanoparticles. |
However, if the synthesis process was not appropriate, the metal contained in the core cannot interact with the reactants because normally metal oxides found on the surface of the size are relatively large, thus blocking the reaction of the reactants with metals contained in the nucleus.19 In this research we modified the solvent for the synthesis of the silver-cerium nanoparticles to produce nano-sized cerium nanoparticles, allowing interaction between reactants and metals in the cell nucleus. Based on the results of XRD and TEM analysis, the silver-cerium particles produced have a particle size diameter of 8.9-12.73 nm. The metal in the core can affect the active metal oxides on the surface so as to increase the selectivity of the bimetal nanoparticles in its application.20
Based on the results of the SEM-EDX analysis in Figure 4, only about 1% of silver metal was present in samples of silver-cerium nanoparticles. This happens because the SEM-EDX analysis was essentially a surface inspection and analysis. The data or appearance obtained was from a surface or layer with a thickness of about 20 micrometers from the surface.21 Based on Fig.3D of cerium silver nanoparticles material in the form of core-shell structure where Ag nanoparticles were present in the core and cerium oxide nanoparticles are on the surface so that a large number of cerium oxides appear in the component composition of cerium silver nanoparticles.
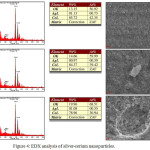 |
Figure 4: EDX analysis of silver-cerium nanoparticles. |
Antibacterial Activity of Silver Nanaoprticles and Silver-Cerium Nanoparticles
Antimicrobial activity testing of silver nanoparticles and silver-cerium nanoparticles colloidal solutions in accordance with the standard method of AATCC147-1998.22 This test is qualitative by measuring how wide the zones of inhibition the media were escherichia coli and staphylococcus aureus that occur after surface contact with paper discs moistened with silver nanoparticle colloid solutions and silver/cerium nanoparticles colloid with variations in concentration was 100, 50, and 25 %.
Figure 5 shown that silver nanoparticles colloid solutions and silver-cerium nanoparticle colloid solutions showed inhibitory activity against Staphylococcus aureus (Gram-positive) (Fig .A and B) and Escherichia coli (Gram-negative) (Fig. C and D) with the formation of zone of inhibition on agar medium containing bacteria even though it had experienced a 25% dilution. The results of this research in accordance with the Sharma et al. 2009 that antibacterial activity of biological synthesized silver nanoparticles was seen against Gram-negative (Pseudomonas putida and Klebsiella pneumonia, E. coli) and (Staphylococcus aureus and Bacillus subtilis) bacteria. Further, the zone of inhibition of silver nanoparticles against Gram-negative and Gram-positive bacteria were measured. The results indicated that silver nanoparticles synthesized from strawberry leaf extract showed effective antibacterial activity both in Gram-negative and Gram-positive bacteria.23
The size zone of inhibition showed the strength of the nanoparticles inhibitory against bacteria. The wider the zone of inhibition caused showed the stronger the inhibitory power of the compound to bacterial growth.24 The width of the zone of inhibition formed using silver colloid solution and silver-cerium colloid solution. The resulting zone of inhibition was measured by a calipers run. The results of the measurement of the zone of inhibition Ag and Ag/Ce nanoparticles shown in Tables 1.
Antibacterial activity of silver nanoparticles and silver-cerium nanoparticles on the growth of Staphylococcus aureus bacteria shown in Figure 5A and 5B. The antibacterial activity of silver nanoparticles and silver-cerium nanoparticles on the growth of Escherichia coli bacteria shown in Figure 5C and 5D. The diameter of the zone of inhibition < 5 mm (weak), a zone of inhibition 5-10 mm (medium), a zone of inhibition 10-20 mm (strong) and very strong ˃20 mm more.25 Based on Table 1, it had shown the diameter of the zone of inhibition % Ag Colloid Ag solution when 100% in S. aureus bacteria 12.5 mm and E. coli 10.97 mm. The category of zone inhibition was strong. The disinfection effect of Ag-doped CeONP was more pronounced on S. aureus than E. coli, although the difference was not wide.
According to Jawetz, et al. (2013) states that antibacterial activity was influenced by 4 factors, namely: extract concentration, a content of metabolite compounds, diffusion power of extract and type of bacteria that are inhibited.26 Antibacterial inhibitory activity test against Gram-positive bacteria (Bacillus cereus) was stronger than Gram-negative bacteria (Escherichia coli) because of the structure of their respective cell walls. Pelczar and Michael (2017), the cell wall structure of Gram-negative bacteria is more complex than the cell wall structure of Gram-positive bacteria. Gram-negative bacteria have cell walls which consist of 3 layers, namely, the outer layer, the middle layer, and the inner layer. The function of the outer layer served as a bacterial barrier to the effects of various antibacterials and in the outer layer had membrane porins that function to prevent the entry of other molecules enter into bacteria.27 Whereas Gram-positive bacteria only have a single layer on the cell wall. Siswandono, 2016 added that the relatively complex Gram-negative bacterial cell wall structure would cause antibacterial compounds to be more difficult to enter cells and find targets for degradation.28
Silver nanoparticles doped with cerium nanoparticles showed an increase in inhibitory with an increase a zone of inhibition after being doped with cerium nanoparticles because the strong disinfection effect of Ag-doped CeONP was attributed to the synergetic combination of two mechanisms, namely, redox catalysis of surface oxygen vacancy.29 Ce in comparison to Ag possess two different oxidation states of Ce3+ (Ce2O3) and Ce4+ (CeO2) and can tolerate free radicals. Normally, it has been observed that Ce was present in CeO2 form at CeO2-nanoparticles surface having deficient oxygen (O) and valency of Ce3+ rather than Ce4+. This defect chemistry in the surface of pre-activated CeO2-nanoparticles can provide the maximum antioxidant potential to them. So, Ce doped Ag can increase the antibacterial activity of nanoparticles.13,30 Based on Table 1, it had shown the diameter of the zone of inhibition Ag-Ce Colloid solution when 100% in S. aureus bacteria 17.34 mm and E. coli 13.52 mm. The category of zone inhibition was strong. The disinfection effect of Ag-doped CeONP was more pronounced on S. aureus than E. coli, although the difference was not wide.
Table 1: The zone of inhibition used Ag and Ag-Ce nanoparticles against bacterial growth.
| No | Concentration | Zone of inhibition used Ag | Zone of inhibition used | ||
| Ag and Ag-Ce nanoparticles solution (%) | nanoparticles | Ag-Ce nanoparticles | |||
| Staphylococcus | Esherichia | Staphylococcus | Esherichia | ||
| aureus | coli | aureus | coli | ||
| 1 | control | 0 | 0 | 0 | 0 |
| 2 | 25 | 8.02 | 0 | 8.25 | 6.75 |
| 3 | 50 | 10.3 | 8,47 | 12.6 | 9.41 |
| 4 | 100 | 12.5 | 10.97 | 17.34 | 13.52 |
The zone of inhibition of Ag-Ce was slightly higher than that of nondoped Ce (table 1) in E. coli and S. aureus. This finding not only justifies the synergetic effect but also supports the conjecture that Ag-doped Ce-nanoparticles produces more oxygen vacancies such that Ag-doped CeONP disinfects better than a simple combination of ceria particles and silver ions. The disinfection effects of Ag-doped CeONP are also superior to a simple combination of undoped CeONP and equivalent Ag+ content.31
Table 2: Synthesis nanoparticles using metal oxide.
| Nanomaterial Oxide | Size (nm) | Antimycobacterial activity | Ref |
| Titanium oxide | 25,7 -47,1 | Effective antibacterial activity for Staphylococcus aureus | 32 |
| Copper Oxide | 23,17 | Potential for external uses as antibacterial agents | 33 |
| Zinc Oxide | 19.6 -20.2 | Antibacterial activity for gram (+ and gram (-) | 34 |
| Silver oxide | 7.0 – 9.0 | Lower when testedagainst E. coli than when tested against S. aureus. | 35 |
| Cerium Oxide | 20.0 – 100.0 | Exhibited inhibition with respect to the gram negative | 36 |
| Titanium oxide-Silver | 15,0 | Higher antibacterial efficacy against E. coli bacteria | 37 |
| Copper | 16.0 – 25.0 | Excellent antibacterial activity against the growth of microorganisms. | 38 |
| Zinc Oxide-Silver | 12,13 | Ag-doped ZnO nanoparticles have ability to destroy bacteria and serve as better antimicrobial agent than ZnO | 39 |
| Silver oxide | 18.61 – 30.01 | Effective antibacterial activity for S. aureus | This work |
| Cerium Silver | oxide- 8.9 – 12.73 | Better antimicrobial agent than AgO | This work |
Synthesis nanoparticles using metal oxide reported in the kinds of literature shown in Table 2 and result of this research are found to be comparable to these report. Accordingly, nanoparticles silver doped cerium oxide can be used and potential for antibacterial effect.
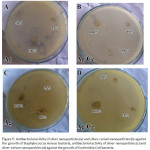 |
Figure 5: Antibacterial activity of silver nanoparticles (a) and silver-cerium nanoparticles (b) against the growth of Staphylococcus Aureus bacteria, antibacterial activity of silver nanoparticles (c) and silver-cerium nanoparticles (d) against the growth of Escherichia Coli bacteria. |
Conclusions
The aim of this research was to made agen antibacteri from silver nanoparticles and silver-cerium nanoparticles. XRD and TEM analysis showed silver-cerium nanoparticles were spherical with the dominant size range of 8.9 -12.73 nm. SEM-EDX analysis showed silver nanoparticles covered by silver nanoparticles that were known as the core-shell structure. Silver nanoparticles doped with cerium nanoparticles showed an increase in inhibitory with an increase a zone of inhibition after being doped with cerium nanoparticles.
Acknowledgements
The authors would like to Government of INDONESIA by Direktorat Jendral Penelitian dan Pengabdian Masyarakat (DRPM) Riset Teknologi dan Pendidikan Tinggi (RISTEKDIKTI), through research schemes Insentif Riset Sistem Inovasi Nasional (INSINAS) for providing funds of this research with the Grant No. 22/INS-1/PPK/E4/2018.
References
- Colussi, S.; Trovarelli, A.; Cristiani, C. ; Lietti, L.; Groppi, G. Catal.Today. 2012, 180, 124-130.
CrossRef - Maqbool, Q.; Nazar, M.; Naz. S.; Hussain, T; Jabeen,N.; Kausar, R.; Anwaar, S.; Abbas, F.; Jan,T. Int. J. Nanomed., 2016, 11, 5015-5025.
CrossRef - Cresi, J.S.; Spadaro, M.C.; D’Addato, S.; Valeri,S.; Amidani,L.; Boscherini,F.; Bertoni,G.; Deiana, D.; Luches,P. Nanotechnology, 2017, 28, 49.
CrossRef - Shimizu, K.; Kawachi, H.; Satsuma, A. Appl.Catal.B: Enviro., 2010, 96,167-175.
CrossRef - Choi, H.W.; Lee, K.H.; Hur, N.H.; Lim, H.B. Anal.Chim.Acta. 2014, 847, 10-15.
CrossRef - Bing, J.; Li, L.; Lan, B.; Liao. G.; Zeng, J.; Zhang, Q.; Li. X. Appl.Catal.B : Enviro, 2012, 115, 16.
- Putri, G.E.; Arief, A.; Jamarun, N.; Gusti, F.R.; Fisli, A. Zilfa, Septiani, U. J.Applicable.Chem., 2017, 6, 1058-1068.
- Vargas, G.O.A; De los Rayes Heredia, J.A.; Montesinos Castellanos, A.; Chen, L.F.; Wang, J.A. Mater. Chem. Phys., 2013, 139, 125.
CrossRef - Jabbar, S.S. Orient.J.Chem., 2018, 34, 2026-2030.
CrossRef - Naik, B.; Hazra, S.; Prasad, V.S.; Ghosh, N.N. Catal. Commun.,2012, 12, 1104.
CrossRef - Chaiyasat, A.; Jearani, S.; Moonmangmee, S.; Moonmangmee, D.; Christopher, L.P.; Alam, M.N.; Chaiyasat, P. Orient.J.Chem., 2018, 34, 1735-1740.
CrossRef - Wang, L.; He,H.; Yu, Y.; Sun, L.; Liu,S.; Zhang, C.; He, L. J. Inorg. Biochem. 2014, 135, 45.
CrossRef - Maqbool ,Q. RSC Adv., 2017, 7, 56575.
CrossRef - Benedetti, F.; Luches, P.; Spadaro, M.C.; Gasperi, G.; D’Addato, S.; Valeri, S.; Boscherini, F. J. Phys. Chem., 2015, 119, 6024−6032.
- Khadar, Y.A; Balamururagan, A.; Devarajan, V.P.; Subramanian, R. Orient.J.Chem., 2017, 33, 2405-2411.
CrossRef - Kayama, T.; Yamazaki, K.; Shinjoh, H. J. Am. Chem. Soc., 2010, 132, 13154.
CrossRef - Singh, G.; Joyce, M.A.; Beddow, J.; Timothy, J.; Mason, J. J. Microbio.Biotech.Food Sci.,2012, 2, 106.
- Mitsudome, T.; Mikami, Y.; Matoba, M.; Mizugaki,T.; Jitsukawa, K.; Kaneda, K. Angew. Chem. Int. Ed., 2012, 51, 136.
CrossRef - Strasser, P.; Koh, S.; Anniyev, T.; Greeley,J.; More,K.; Yu, C.F.; Liu, Z.C.; Kaya, S.; Nordlund, D.; Ogasawara, H.; Toney, M.F.; Nilsson, A. Nat. Chem. 2010, 2, 454.
CrossRef - Ghorbani, H.R. Orient.J.Chem. 2014, 30, 1941-1949.
CrossRef - Sen, P.; Ghosh,J.; Abdullah,A.; Kumar, P. Vanada. Proc. Indian Acad.Sci.(Chem. Sci.), 2003, 115, 499-508.
CrossRef - Singh, H.; Du, J.; Yi, T.H. Artif. Cells Nanomed.Biotech., 2017. 45, 1310.
CrossRef - Sharma, V.K.; Yngard, R.A.; Lin, Y. Adv Colloid Interface Sci., 2009, 145, 83-96.
CrossRef - Shin, S.H.; Kim, Y.H.; Park, W.K.; Jung, H.C.; Koo, K.W.; Kim; Sook. Appl. Environ. Microbiol., 2008, 74, 2171.
- Davis, W.W and Stout, T.R. Appl Microbiol. 1971, 22, 659-665.
- Jawetz, Melnick, Adelberg’s. Medical Microbiology. Twenty-Sixth Edition. Geo. F. Brooks, MD. 2013. Professor of Laboratory Medicine. a LANGE Medical Book.
- Pelczar, Michael, J. Microbio., 2017, 7th edition, McGraw-Hill Companies, New York.
- Siswandono. Kimia Medisinal, 2016, 2, Airlangga University Press. Indonesia.
- Feng, Q.L.; Wu,.; Chen, G.Q.; Cui,F.Z.; Kim,T.N.; Kim, J.O. J.Biomed.Mater.Res., 2000, 52, 662–6688.
CrossRef - Bae, E.; Park, H.J.; Park, j.; Yoon, J.; Kim, Y.; Choi, K.; Yi, J. Bull. Korean Chem. Soc. 2011, 32, 613-619.
CrossRef - Tsai, D.S.; Yang, T.S.; Huang, Y.S.; Peng, P.W.; Ou, K.L. Inter.J. Nanomed., 2016, 1, 2531-2542.
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Bai, Y.; Li, K. Progr.Organic Coat., 2012, 73, 219-224.
CrossRef - Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.A.; Karuppiah, P.; Al-Dhabi, N.A. J.Nanomater., 2014, 637858, 4.
- Shahid, S.; Khan, S.A.; Ahmad, W.; Fatima, W.; Knawal, S.; Indian J.Pharm Sci, 2018, 80,173-180
CrossRef - Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park,Y.H.; Hwang, C.; Kim, Y.; Lee, Y.; Jeong, D.H.; Cho, M. Nanomed. Nanotech. Bio. Med., 2014, 10, 1119.
CrossRef - P. Reshma, K. Ashwini. J Nanomater Mol Nanotechnol., 2017, 6, 3.
CrossRef - Thiel, J.; Pakstis, L.; Buzby, S.; Raffi, M.; Ni,C.; Pochan, D.J.; Ismat Shah, S. Small Journal, 2017, 3, 799-803.
CrossRef - Sasikala, R.; Kutti Rani, S; Karthikeyan, K.; Easwaramoorthy, D. DJ J.Eng.Chem.Fuel, 2016, 1, 43-51.
CrossRef - Dowlatababdi,F.H.; Amiri, A.; Sichani,M.M. Nanomed. J., 2017, 4,50-54.

This work is licensed under a Creative Commons Attribution 4.0 International License.









