The Synthesis of a Novel Tröger Base Polymer of 2,6(7)-Diamino-9,9,10,10-Tetramethyl-9,10-Dihydroanthracene
Chemistry Department, College of Science for Women, University of Babylon, Iraq.
Corresponding Author E-mail: sadiqkarim77@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340559
Article Received on : 29-04-2018
Article Accepted on : 27-08-2018
Article Published : 11 Oct 2018
Condensation polymerisation technique has been employed to synthesise a Novel Tröger base polymer with thermal stability and microporosity. The synthesis process starts with alkylating anthracene, then nitrating and reducing this to produce the monomer. A Tröger base polymer is obtained by polymerising the monomer to afford a white polymer with good solubility into dichloromethane and chloroform, good thermal stability to ~377oC and a good BET surface area of 368.6 m2/g with a total pore volume of 0.4166 ml/g.
KEYWORDS:Heterocyclic Polymer; Tröger Base Polymer; Tröger Base Polymer Of 2,6(7)-Diamino-9,9,10,10-Tetramethyl-9,10-Dihydroanthracene
Download this article as:| Copy the following to cite this article: Karim S.A. The Synthesis of a Novel Tröger Base Polymer of 2,6(7)-Diamino-9,9,10,10-Tetramethyl-9,10-Dihydroanthracene. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Karim S.A. The Synthesis of a Novel Tröger Base Polymer of 2,6(7)-Diamino-9,9,10,10-Tetramethyl-9,10-Dihydroanthracene. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50537 |
Introduction
The German chemist (Julius Tröger) during his Ph.D., studied, synthesised and isolated (2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine) also known as Tröger base 1 from the condensation of methanal (formaldehyde) with 4-aminotoluene (p-toluidine) in an acid-catalysed media.1 Through a chemical reaction, 2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine structure2 was confirmed by M. A. Spielman and then later by single crystal X-ray diffraction.3 After a decade, the Tröger base was described as “fascinating molecules”,4-7 because their structure in which the chiral 1,5-diazocine bridge locks contains two stereogenic nitrogen atoms in its rigid twisted V-shape,8-10 besides the two enantiomers of the Tröger base which exist at room temperature.11-12 Some years ago, a patent application was made to prepare Tröger base polymers using aromatic monomers containing diamine with methylene supplier as dimethoxymethane in trifluoroacetic acid.13 Subsequently, several Tröger bases synthesised as ladder polymers then tested as potential applications for gas separation membranes14-20 or as network polymers.21 Furthermore, Tröger base polymers are used in various applications as heterogeneous catalysis.22-24 Recently, Tröger base polymer was synthesised by condensation of 2,8-disubstituted Tröger base monomers with certain aromatic compounds.25 In this work a Tröger base is prepared for an anthracene derivative after alkylation, nitration and reduction to facilitate the monomer, then the polymerisation of it to form the polymer. This polymer shows good thermal stability and microporosity.
Materials and Method Materials and Equipment
Diglyme, LiAlH4 and Raney®-Nickel were purchased from Sigma-Aldrich. Anthracene, Hydrazine monohydrate, Dimethoxymethane, Acetonitrile and Potassium nitrate were purchased from Alfa Aesar. Trifluoroacetic acid and Trifluoroacetic anhydride were supplied by Fluorochem.
Equipment used in this research
Melting points (Mp) were recorded using a Sturat Melting Point SMP10 apparatus. Fourier transform infrared adsorption spectra (FTIR) were recorded in the range 4000-400 cm-1 using an FTIR spectrophotometer as a solid (powder) by Shimadzu IRAffinity-1S. Nuclear Magnetic Resonance (NMR): 1H and 13C and NMR spectra were recorded in a suitable deuterated solvent using Bruker Ascend TM 500 at the School of Chemistry, University of Edinburgh. Mass Spectrometry: Low-resolution mass spectrometric (LRMS) and high-resolution mass spectrometric results (HRMS) were obtained using a Thermo MAT 900 XP, double focusing sector at The University of Edinburgh. Gel Permeation Chromatography (GPC) analyses were performed on chloroform solutions (2 mg ml-1) using a GPC MAX variable loop equipped with two KF-805L SHODEX columns and a RI(VE3580) detector, operating at a flow rate of 1 ml min-1. Calibration was achieved using Viscotek polystyrene standards (Mw 1000 – 1,000,000 g mol-1). BET Surface Areas: Low-temperature (77 K) nitrogen adsorption/desorption isotherms were obtained using Coulter SA3100 surface area analyser instruments. Thermo-gravimetric analyses were performed on a Thermal Analysis SDT Q600 system, heating samples (~10 mg) at a rate of 10 °C/min from 50°C to 800°C under a nitrogen atmosphere.
Procedures Synthesis route of 2,6(7)-diamino -9, 9,10,10-tetramethyl-9,10-dihydroanthracene- Tröger base polymer (DATMA-TB) Synthesis of 9, 9,10,10-tetramethyl-9,10-dihydroanthracene (TMA)
According to relevant literature26, in a nitrogen atmosphere, a mixture of Anthracene (18.71 g, 105.0 mmol), LiAlH4 (10.00 g 270.30 mmol) and diglyme (294.00 ml, 2.07 mol) was prepared in a 1L round-bottom flask. Then the mixture was refluxed at 150oC for 10 hours, after which time the reaction was cooled to room temperature and the mixture was poured into 2L (1N) HCl and stirred for 30 minutes. The organic layer was extract by ethyl acetate and recrystallization with ethanol to produce 9,9,10,10-tetramethyl-9,10-dihydroanthracene 7.50 g, 30 % as a white powder; mp = 166-168oC; FTIR (solid) n = 3055 cm-1 (str. aromatic C-H) , 2972 & 2963 cm-1 (asy. and sy. str. of CH3 group), 1362 cm-1 (sy. bending of CH3); 1H NMR (500 MHz, CDCl3) δ 8.13 (d, J = 8.8 Hz, 4H, ArH), 7.70 (d, J = 8.7 Hz, 4H, ArH), 1.76 (s, 12H, CH3); 13C NMR (126 MHz, CDCl3) δ 142.7 (Ar), 122.4 (Ar), 121.7 (Ar), 38.5 (quadruple carbon), 34.5 (CH3); LRMS (EI, m\z): calculated for C18H20 236.3, 236.4 (M+) found.
Synthesis of 9,9,10,10-tetramethyl-2,6(7)-Dinitro-9,10-dihydroanthracene (DNTMA)
A mixture of TMA (5.00 g, 21.15 mmol), potassium nitrate (KNO3) (4.28 g, 42.30 mmol) and Acetonitrile (250.00 ml) was prepared. Then trifluoroacetic anhydride (TFAA) (35.00 ml, 52.05 g, and 247.80 mmol) was added drop-wise. The mixture was then stirred for 24 hours at room temperature, after which a white precipitate had formed. The solvent was removed using a vacuum pump and the residue was stirred with water then extracted with chloroform to produce a yellow solid. This crude product was subjected to column chromatography (eluent: Chloroform) to produce 9,9,10,10-tetramethyl-2,6(7)-Dinitro-9,10-dihydroanthracene (DNTMA) 4.20 g, 60 % as an off-white; mp = 236-238o C; FTIR (solid) n = 1516 and 1346 cm-1 for asymmetric and symmetric for NO2 group; 1H NMR (500 MHz, CDCl3) δ 8.13 (d, J = 8.8 Hz, 2H, ArH), 7.71 (d, J = 8.8 Hz, 2H, ArH), 7.69 (s, 2H, ArH), 1.76 (s, 12H, CH3) ; 13C NMR (126 MHz, CDCl3) δ 148.0 (Ar), 147.0 (Ar), 128.4 (Ar), 126.8 (Ar), 122.4 (Ar), 121.6 (Ar), 38.5 (quadruple carbon), 34.7 (CH3); HRMs (EI, m\z): calculated for C19H22N2O4 326.40: 326.2950 (M+) found.
Synthesis of 2,6(7)-Diamino-9,9,10,10-tetramethyl-9,10-dihydroanthracene (DATMA)
A suspension of 2,6(7)-Diamino-9,9,10,10-tetramethyl-9,10-dihydroanthracene (DNTMA) (5.00 g, 15.32 mmol) in THF (200 ml) was stirred for 30 minutes in a nitrogen atmosphere. Then Raney Nickel (40 mg) was added to the mixture. Hydrazine monohydrate (13.00 g, 259.69 mmol) was added drop-wise and the mixture was refluxed for 24 hours. The colourless mixture was cooled in an ice bath and filtered in nitrogen. The organic layer was extracted with diethyl ether and the solvent was removed in a vacuum at 25 oC to produce 2,6(7)-Diamino-9,9,10,10-tetramethyl-9,10-dihydroanthracene 3.1 g, 90% as a white powder; mp = 202-205o C; FTIR solid n = 3340 & 3309 cm-1 for asy. & sy. of NH2 group, 1620 cm-1 bending vibration of NH2 group; 1H NMR (500 MHz, CDCl3) δ 7.31 (d, J = 3.2 Hz, 2H, Ar), 7.29 (d, J = 3.2 Hz, 2H, Ar), 6.80 (s, 2H, Ar), 3.59 (s, 4H, NH2), 2.17 (s, 12H, CH3); 13C NMR (126 MHz, CDCl3) 145.3 (Ar), 143.1 (Ar), 128.2 (Ar), 119.0 (Ar), 114.8 (Ar), 113.0 (Ar), 37.1 (quadruple carbon), 35.4 (CH3); HRMs (EI, m\z): calculated for C19H26N2 282.4300: 266.2987 (M+) found.
Synthesis of 2, 6(7)-Diamino-9,9,10,10-tetramethyl-9,10-dihydroanthracene-Tröger base polymer (DATMA-TB)
9,9,10,10-Tetramethyl-2,6(7)-diamino-9,10-dihydroanthracene (DATMA) (1 g, 3.76 mmol) was dissolved in dimethoxymethane (DMM) (2 ml, 1.72 g, 23.24 mmol). The solution was cooled in an ice bath and trifluoroacetic acid (10 ml) was added drop-wise for 10 minutes and the mixture was stirred for 7 days at room temperature. The viscous mixture was slowly poured into 25 ml of aqueous ammonium hydroxide solution and stirred vigorously for 2 hours during which a solid was formed. The solid was collected by filtration, washed with water and methanol until the washed liquids ran clear. The resulting powder was dissolved in chloroform and re-precipitated with methanol three times and then dissolved in chloroform. The solution was added drop-wise into hexane with vigorous stirring. The polymer was collected by filtration, as a fine powder. The polymer was dried in a vacuum oven at 120°C for 5 hours to produce 0.84 g, 70% as an off-white powder; FTIR (solid) (Fig. 1) n = 3055 cm-1 (Ar-H), 2943 cm-1 (asy. str. CH3), 2963 cm-1 (asy. & sy. str. of CH2 & CH3 groups), 2860 cm-1 (sy. str. of CH2 group), 1680, 1597 and 1489 cm-1 (C=C of Ar), 1325 cm-1(Ar-N), 1215 cm-1 (Caliphatic-N); 1H NMR (500 MHz, CDCl3) (Fig. 2). δ 7.89 (br. m, 4H, Ar), 4.48 (br. d, J = 17.0 Hz, 2H, aliphatic), 4.27 (br. s, 2H, aliphatic), 3.90 (br. d, J = 17.0 Hz, 2H, aliphatic), 1.73 (br. s, 12H, CH3); 13C NMR (126 MHz, CDCl3) (Fig. 3). δ 146.9 (Ar), 144.5 (Ar), 141.2 (Ar), 132.5 (Ar), 131.4 (Ar), 127.9 (Ar), 126.6 (Ar), 126.4 (Ar), 125.9 (Ar), 67.4 (aliphatic), 64.24 (aliphatic), 55.0 (aliphatic), 17.4 (CH3). On a BET surface area (Fig. 4), the sample was degassed overnight before analysis to give a value of 368.6 m2/g with a total pore volume = 0.4166 ml/g; Gel permeation chromatography (GPC) (Fig. 5), polystyrene standards (Mw 1000–1000000 g mol-1): Mn=11400, Mw=19400 g/mol. The TGA analysis (Fig. 6) of that polymer was the initial weight loss due to thermal degradation commencing at ~ 377.53°C with an 1.28 % loss of mass.
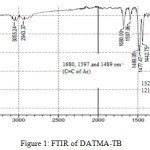 |
Figure 1: FTIR of DATMA-TB. |
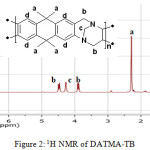 |
Figure 2: 1H NMR of DATMA-TB |
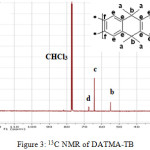 |
Figure 3: 13C NMR of DATMA-TB |
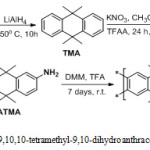 |
Scheme 1: synthesis of 2, 6(7)-Diamino-9,9,10,10-tetramethyl-9,10-dihydroanthracene-Tröger base polymer (DATMA-TB). |
Results and Discussion
A TMA is synthesised by alkylation of anthracene, with a lower yield. This low yield can be attributed to several products of alkyl anthracene which were formed, 30% of which was tetramethyl. This alkylation process occurs with LiAlH4 as a reducing agent to react with diglyme to form an intermediate from a methyl carbonium ion to attack the active positions of anthracene. A DNTMA is synthesised by nitration of TMA, which forms mixed compounds of the 2,6 and 2,7 dinitro position. Potassium nitrate and the trifluoroacetic anhydride were used to form the nitrating agent trifluoroacetyl nitrate, potassium nitrate being chosen because it is the least hydroscopic compared with other common nitrates while the nitric acid produces nitro compound multiproducts. This method affords a good product yield with small quantities of impurities which were easily separated. The nitration was carried out in acetonitrile solvent because of the greater solubility of the inorganic salts. The reaction was processed at room temperature because increased heating will result in a higher ratio of impurity. The crude product was subjected to column chromatography (eluent: Chloroform) to produce as an off-white powder. The FTIR of DNTMA appears as asymmetric and symmetric stretching of NO2 1516 and 1346 cm-1, respectively. The 13C NMR of DNTMA refers to Caromatic-NO2 at δ 148.0 and 147.0. The reduction of DNTMA to DATMA using hydrazine monohydrate and a catalytic quantity of Raney nickel produced amines with the highest purity. The reduction was carried out under reflux to decrease the reaction time. The reduction must be conducted under an inert atmosphere using solvents that have been thoroughly deoxygenated. The FTIR of DATMA appears as asymmetric and symmetric stretching of the NH2 group at 3340 & 3309 cm-1, respectively, with bending vibration of the NH2 group at 1620 cm-1. The 1H NMR of DATMA refers to the NH2 group at δ 3.59 ppm. The 13C NMR of DATMA refers to Caromatic-NH2 at 145.3 and 143.1 ppm. The DATMA monomer was polymerised using dimethoxymethane (DMM) which is a “methylene” source in a strongly acidic solvent, and a catalyst such as trifluoroacetic acid (TFA). A typical procedure of polymerisation is one equivalent of a pure aromatic diamine mixed with five equivalents of dimethoxymethane (excess) and cooled to the temperature of ice. TFA (8-10 ml per gram of monomer) is then slowly added drop-wise over a period of 10 to 30 minutes. The polymerisation mixture is stirred at room temperature under a constant inert atmosphere until the solution realises the required viscosity. The colour of the solution will change to dark red. Tröger base polymerisation exhibits the rate of acid addition and changes in concentration. To quench it, the mixture is slowly poured into aqueous ammonium hydroxide solution to precipitate the polymer as an amorphous solid. The FT-IR of polymer (Fig. 1) shows disappearing vibration stretching and bending of –NH2 and the appearance of a new vibration of Caliphatic-N for Tröger-base cyclic. 1H NMR (Fig. 2) clearly refers to Ar-N-CH2 for Tröger-base cyclic at δ 4.48 and 3.90 ppm beside to N-CH2-N at δ 4.27 ppm. 13C NMR (Fig. 3) of polymer refers to Caromatic-N at δ 146.9 and 144.5 ppm and Caliphatic-N at δ 67.4 and 64.24 ppm. The solubility of that polymer is shown as good in DMSO, DMAC, NMP, DMF, DCM and CHCl3 but insoluble in Toluene, Xylene, THF, ethanol and acetone (Table 1). The BET surface area calculated from nitrogen isotherms shows microporosity of DATMA-TB polymer where the BET surface area is 368.6 m2/g, the total pore volume = 0.4166 ml/g, which is attributed to methyl groups besides the heterocyclic V shape of the Tröger base BET surface area (Fig. 4). Gel permeation chromatography (GPC) (Fig. 5) polystyrene standards (Mw 1000–1000000 g mol-1) Mn=11400, Mw=19400 g/mol. Thermal gravimetric analysis (TGA) of the DATMA-TB polymer showed an initial weight loss due to thermal degradation commencing at ~ 377°C with a 1.28 % loss of mass, then a decrease in mass until a loss of ~ 47% at ~ 631°C where complete decomposition took place (Fig. 6).
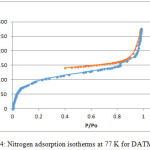 |
Figure 4: Nitrogen adsorption isotherms at 77 K for DATMA-TB |
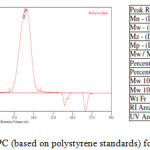 |
Figure 5: GPC (based on polystyrene standards) for DATMA-TB. |
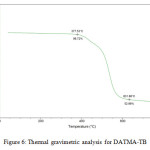 |
Figure 6: Thermal gravimetric analysis for DATMA-TB. |
Table 1: solubility of DATMA-TB in common organic solvents
|
Solvents I,II |
|||||||||||
| DATMA-TB | DMSO | DMAC | NMP | DMF | Toluene | Xylene | THF | Acetone | DCM | CHCl3 | EtOH |
|
+ |
+ |
+ |
+ |
– |
– |
– |
– |
+ |
+ |
– |
|
I Solubility guide: (+) soluble, (-) insoluble. II All solvents tested had a concentration of 0.01 g ml-1 at room temperature.
Conclusion
In summary, TMA synthesis by alkylating anthracene took place with a 30% yield following nitration to produce DNTMA 60%, the DATMA resulting in 90%. DATMA-Tröger base polymer was produced as white by condensation polymerisation of DATMA with DMM in TFA. TMA, DNTMA and DAMTA compounds are characterised by FT-IR, 1H-NMR, 13C-NMR besides TGA and the BET surface area of polymer.
Reference
- Tröger, J. J. für Praktische Chemie. 1887, 36, 225-245.
- Spielman, M. A.; J. Am. Chem. Soc. 1935, 57, 583-585.
CrossRef - Larson, S. B.; Wilcox; C. S., Acta Cryst. Section C. 1986, 42, 224-227.
CrossRef - Vögtle, F., Fascinating Molecules in Organic Chemistry; Wiley and Sons. 1992, 237-249.
- Becker D. P.; Finnegan P. M.; Collins P. W.; Tetrahedron Letter. 1993, 34, 1889-1892.
CrossRef - Adrian, J. C.; Wilcox C. S.; J. Am. Chem. Soc. 1992, 114, 1398-1403.
CrossRef - Weber , E.; Müller, U.; Worsch, D.; Vögtle, F.; Will, G.; Kirfel, A. ; J. Chem. Soc. Chem. Comm. 1985, 1578-1580.
- Sergeyev, S. Helv. Chim. Acta. 2009, 92, 415-444.
CrossRef - Adrian, J. C. , Wilcox, C. S. ; J. Am. Chem. Soc. 1989, 111, 8055-8057.
CrossRef - Wilcox, C.S.; Cowart, M. D. ; Tetrahedron Letters. 1986, 27, 5563-5566.
CrossRef - Didier, D.; Tylleman, B.; Lambert N.; Velde, C.; Blockhuys F.; Collas A.; Sergeyev S. Tetrahedron. 2008, 64, 6252-6262.
CrossRef - Trapp, O.; Schurig V. J. Am. Chem. Soc. 2000, 122, 1424-1430.
CrossRef - Carta, M.; Croad M.; McKeown N. B. 2010, WO2012/035328 A1.
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen J. C.; Bernardo P.; Bazzarelli, F; McKeown N. B. SCIENCE. 2013, 339, 303-307.
CrossRef - Carta M.; Croad M.; Malpass-Evans R.; Jansen J. C.; Bernardo P.; Clarizia,G.; Lanc, K. ; McKeown N. B. Adv. Mater. 2014, 26, 3526-3531.
CrossRef - Rose I.; Carta M.; Malpass-Evans R.; Ferrari M.; Bernardo P.; Clarizia G.; Jansen J. C.; McKeown, N. B. ACS Macro Letters. 2015, 4, 912-915.
CrossRef - Z. G. Wang, X. Liu, D. Wang and J. Jin, Poly. Chem. 2014, 5, 2793-2800.
CrossRef - Zhuang Y.; Seong, J. G.; Do, Y. S.; Lee, W. H.; Lee, M. J.; Cui, Z.; Lozano, A. E.; Guiver, M. D.; Lee, Y. M. Chem. Comm. 2016, 52, 3817-3820.
CrossRef - Ghanem, B.; Alaslai N.; Miao, X.; Pinnau I.; Polymer. 2016, 96, 13-19.
CrossRef - Karim S. A.; Braihi A. J. JGPT. 2017, 12, 9, 30-35.
- Carta M.; Croad; M.; Bugler K.; Masyib, K. J; McKeown N. B. Polym. Chem. 2014, 5, 5262-5266.
CrossRef - Rong Y.; Malpass-Evans R.; Carta M.; McKeown, N. B.; Attard G. A.; Marken, F. Electroanalysis. 2014, 26, 904-909.
CrossRef - Cabrero-Antonino J. R.; García T.; Rubio-Marqués P.; Vidal-Moya J. A.; Leyva-Pérez A.; Al-Deyab S. S.; Al-Resayes S. I.; Díaz U.; Corma A. ACS Catal. 2011, 1, 147-158.
CrossRef - Poli E.; Merino E.; Diaz U.; Brunel, D.; Corma A. J. Phys. Chem. C. 2011, 115, 7573-7585.
CrossRef - Li W.; Michinobu T. Macromol. Chem. and Phys. 2016, 217, 863-870.
CrossRef - Kamatani J.; Takiguchi T.; Okada S. US Patent Application. 2008, 20080007161.

This work is licensed under a Creative Commons Attribution 4.0 International License.










