Synthesis, Characterization of SiO2/TiO2 and SiO2/Al2O3 Nano-Composites for the Photo-Degradation of Acid Brown- 43 dye with Irradiation of Solar Light
P. Thirumavalavan1,2 , Venckatesh Rajendran2 and Rajeshwari Sivaraj2
, Venckatesh Rajendran2 and Rajeshwari Sivaraj2
1Research and Development Centre, Bharathiar University, Coimbatore – 641 046, India.
2Department of Chemistry, Government Arts College, Udumalpet – 642 126, India.
Corresponding Author E-mail: thirumap@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340555
Article Received on : 14-07-2018
Article Accepted on : 30-08-2018
Article Published : 12 Oct 2018
In this study we have found that photo degradation of Acid brown- 43 dye with irradiation Solar light Using synthesized SiO2/ TiO2 and SiO2/Al2O3 Nano-composites which prepared by chemical precipitation technique using microwave irradiation. The structure and morphology of SiO2/TiO2 and SiO2/Al2O3) Nano composites were characterized by SEM, EDAX & TEM analysis. The similarities in photo degradation mechanism, SiO2 , Al2O3 and TiO2 have as good as band gap energy, and possess worthy standing as photo-catalysts. SiO2/TiO2 and SiO2/Al2O3 nanoparticles have large surface area and thus provide a large number of active sites for interaction among the particles of different oxides. This synthesized Nano-composites of SiO2/TiO2 and SiO2/Al2O3 photo-catalyst sample showed tremendous photo-catalytic activity for the degradation of Acid Blue - 43 under direct exposure to solar irradiation with respect to exposed time and dose of Nano-composites.
KEYWORDS:Acid brown– 43; Nano-Composite; Photo-Degradation; Solar Irradiation
Download this article as:| Copy the following to cite this article: Thirumavalavan P, Rajendran V, Sivaraj R. Synthesis, Characterization of SiO2/TiO2 and SiO2/Al2O3 Nano-Composites for the Photo-Degradation of Acid Brown- 43 dye with Irradiation of Solar Light. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Thirumavalavan P, Rajendran V, Sivaraj R. Synthesis, Characterization of SiO2/TiO2 and SiO2/Al2O3 Nano-Composites for the Photo-Degradation of Acid Brown- 43 dye with Irradiation of Solar Light. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50669 |
Introduction
Now a days, photocatalysis of semiconductors havingconcerned a countless deals of investigationowing to its possible application to solve environmental difficulties. Subsequently a photocatalytic practice is based on the generation of electron/hole pairs by means of band-gap radiation; the coupling of dissimilar semiconductor metal-oxides seems beneficial to attain a additional efficient electron-hole pair separation under irradiation and a higher photocatalytic activity.1 Nanoparticles of Metal-Oxide attract countlessconsideration in current years an account of their superior electronic and chemical properties. TiO2 is largely used as photocatalyst due to its high photocatalytic efficiency, physical and chemical stability, low cost and toxicity. In recent yearssome research papers concerning the enhancing of TiO2 photocatalytic activity have been described.2-4 So manyconcerns modifying the photocatalyst by coupling TiO2 to other metal-oxides. ZnO could be used as a photocatalyst and has drawn enhancingconsiderationdue to its photocatalytic efficiency is comparable to that of TiO2.5
Last few decades numerous research effects in the field of photocatalysis by semiconducting materials through particular system have been studied. Textile dye produces huge amount of polluted effluents that are usually discharged on the fresh water bodies and ground water aquifers.6 This effluent water causes damages to the ecological system of the receiving surface water capacity and certain a lot of disturbance to the surface and ground water resources. Maximum of the dyes used in the cloth industries are constant to light and biodegradable. In order to decrease the danger of environmental contamination from such as effluent water, it is needed it purify them earlier discharging it into the surroundings.7-9
Though the aqueous phase photochemical degradation of Acid Brown-43 has been investigated under the condition of semi-conducting oxides like ZnO/TiO2,there are numerous techniques of nano particles creation such as organometallic, sol-gel, microwave methods and hydrothermal techniques.10 In the current research, ZnO/TiO2 nano composites were synthesized with help of microwave radiation. Since the intense friction and the collision of molecules produced by microwave treatment, microwave radiation not only offers the energy for reheating but also speed up the nucleation. With microwave radiation on the reactant solution, concentration gradients and temperature could be controlled leading to even nucleation.11 Microwave-based production technique is one of the simplest, energy-saving, green and rapid techniques for huge scale creation of nano materials. Microwave production is the innovative method of synthesis of nano metal oxide semiconductor which is sanitary, eco-friendly, commercial, energy-efficient, quick and suitable technique of heating and outcomes in high quantity in shorter reaction periods.12
In this work, the materials which were utilized for the creation of metal oxide nanoparticles TiO2SiO2 and Al2O3 and the methods of preparation of SiO2/TiO2 and SiO2/Al2O3 nano-composites. The instrumental practices used to the characterization of these Nano-materials and the procedure agreed to study the elimination of colour dyes by photo-degradation under various conditions such as different time interval irradiation and difference concentration of nano composite dosage are described in detail. It has been found that the synthesized SiO2/TiO2 and SiO2/Al2O3 Nano-composite of photo-catalyst sample showed tremendous photocatalytic activity for the degradation of Acid Brown – 43 under straight exposure to solar irradiation with respect to exposed time and dose of nanocomposites.
Materials and Methods
Preparation of SiO2/TiO2 and SiO2/Al2O3 Nanocomposites
SiO2/TiO2 and SiO2/Al2O3 nanocomposites were synthesized using the following procedure. Sol-gel technique was successfully adopted for synthesis of TiO2 nanoparticles. TTIP (Titanium tetra isopropoxide) was used as precursor; HCl was mixed with ethanol and was stirred for ten minutes. To this mixture TTIP was added in the ratio of 1:4:2 and the stirring were continued for 1 h at room temperature. Then 50 ml of deionized water was added, the temperature was raised to50°C and stirred for 3 hrs until the solution changed into colorless TiO2 gel.
SiO2 sol was prepared by mixing 6 gm of silicic acid with 40ml of THF in the ratio of 1:2 and this SiO2 sol was added drop-wise to the TiO2 gel, which resulted in yellowish brown solution. This mixture was stirred for 3 h at room temperature, then it was raised to 80°C and the stirring was continued for an hour. Finally, yellowish brown color changed to yellow and the product was dried in microwave oven at about 100°C for one hour. Finally, the composite was calcined at 400°C.13
SiO2 sol was mixed drop-wise to aluminium chloride with HCl and deionized water was added and the procedure was repeated as above.
Preparation of the dye solution
The dyes used for the present study were AB-43. All the dyes used were of analytical grade and purchased. The stock solutions were prepared by dissolving 1000 mg of dye in 1litre of deionized water. Other concentrations (10, 20, 30 and 40 ppm) were prepared from stock solutions by appropriate dilution with double distilled water. The pH of the solution was adjusted to desired values by adding 0.1 M HCl or 0.1 M NaOH. Fresh dilutions were used for each experiment.
Chemical structure of AB-43
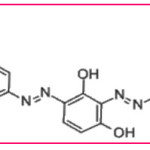 |
Scheme 1 |
Instruments and Chemicals Used for Analysis
Sample colour was analyzed by Spectrophotometer (Genesys10uv, Thermo Electron Corporation). The synthesized nano-composites were scanned by the SEM (JOEL, JSM-3690LV). Additionally the qualitative elemental analysis i.e. Energy Dispersive Analysis of X-Ray (EDAX) was also done by JOEL, JED-2300 analyzer, which would provide the distribution of elemental composition on the surface. The synthesized nano-composites were viewed at high magnification using TEM, JEOL-TEM2100.All the chemicals used were of analytical grade and used as purchased. Titanium tetra isopropoxide, silicic acid, THF, ethanol, isopropyl alcohol, hydrochloric acid and Aluminium chloride were purchased from Merck and Acid Brown-43 dye was purchased from a local firm. Double distilled water was used throughout the studies for the preparation of aqueous solution.
Photocatalytic Activity Evaluation of SiO2/TiO2 and SiO2/Al2O3
Photocatalytic activity of the synthesized nano-composites was evaluated by the degradation of aqueous solution of AB-43. All of the experiments were conducted in an opened Pyrex vessel of 250 ml and in identical conditions. The various concentrations of photocatalyst were placed in a different vessel. Prior to irradiation the suspension was manually stirred in the sunlight for 30 – 240 minutes time variations. During irradiation agitation was maintained to keep the suspension homogeneous. The suspension was sampled at regular intervals and immediately centrifuged to remove catalyst particles. The concentration of the AB-43 dye before and after reaction were measured by means of a UV-Visible spectrophotometer at wavelength 445nm.The Photocatalytic degradation efficiency of the dye solution was calculated using the following equation. The efficiency of the catalyst was calculated using the following equation.
R (%) = (A0 – (A/A0)) × 100
Where A0 is the absorbance of dye solution before the illumination, A is the absorbance of dye solution after degradation.14
Morphological Studies
Scanning Electron Microscope and EDAX
Scanning electron microscopy is a technique for great resolution surface imaging. The SEM uses an electron beam for surface imaging. The advantages of SEM over light microscopy are greater magnification and much larger depth of field. Different elements and surface topographies emit different quantity of electrons, due to which the contrast in SEM micrograph is representative of the surface topography (Fig 1 & 2). The EDAX analysis of the sample was shown in the Figs 5 & 6. The SEM image of SiO2/TiO2 nano-composite SiO2 with thickly covered on TiO2. The SiO2/TiO2 nano-composite had spherical shapes and some of the TiO2 nanoparticles agglomerated were also seen in SEM image. EDAX analysis confirmed the presence of metal oxide bonds in the nanoparticles presence of Si, Ti and O in the nano composite. The SEM image of SiO2/Al2O3 nano-composite showed SiO2 with thickly covered on Al2O3. The elements present in EDAX microanalysis spectrum confirmed the presence of Si, Al and O in the nano-composite.
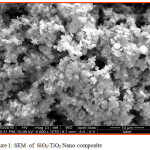 |
Figure 1: SEM of SiO2/TiO2 Nano-composite. |
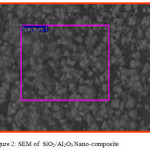 |
Figure 2: SEM of SiO2/Al2O3 Nano-composite. |
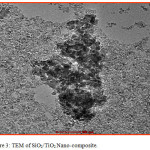 |
Figure 3: TEM of SiO2/TiO2 Nano-composite. |
 |
Figure 4: TEM of SiO2/Al2O3 Nano-composite. |
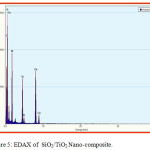 |
Figure 5: EDAX of SiO2/TiO2 Nano-composite. |
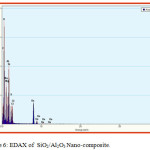 |
Figure 6: EDAX of SiO2/Al2O3 Nano-composite. |
Transmission Electron microscope
Transmission electron microscopy (TEM) is an imaging technique whereby a beam of electrons is focused onto a specimen causing an enlarged version to appear on a fluorescent screen or layer of photographic film, or to be detected by a CCD camera. The microstructure, e.g. the grain size, SAED (Selected Area Electron Diffraction) patterns and lattice defects were studied by use of the image mode, while the crystalline structure was studied by the diffraction mode. A small amount of powder samples were re-dispersed in ethanol, solicited, deposited on a copper grid, air dried and used for TEM analysis (Fig 3 & 4). TEM strongly supported the nano phase formation of SiO2/TiO2 and SiO2/Al2O3 nano-composites.
Result and Discussion
Table 1: Degradation of AB-43 dye color by nano-composite of SiO2/TiO2 and SiO2/Al2O3 with respect to time and concentration of nano-composite.
| Nano composite | Agitation time (min) / Dose (mg/l) | 10 (mg/l) | 20 (mg/l) | 30 (mg/l) | 40 (mg/l) |
| % of degradation | % of degradation | % of degradation | % of degradation | ||
| SiO2/TiO2 | 30 | 70.8 | 65.9 | 59.2 | 45.3 |
| 60 | 88.6 | 78.4 | 75.6 | 59.6 | |
| 90 | 97.2 | 88.6 | 85.4 | 75.1 | |
| 120 | 99.8 | 93.4 | 91.1 | 89.4 | |
| 150 | 99.8 | 99.2 | 97.5 | 92.7 | |
| 180 | 99.8 | 99.2 | 98 | 94.2 | |
| 210 | 99.8 | 99.2 | 98 | 94.2 | |
| 240 | 99.8 | 99.2 | 98 | 94.2 | |
| SiO2/Al2O3 | 30 | 72.2 | 67.3 | 64.2 | 59.8 |
| 60 | 80.9 | 78.6 | 72.8 | 65.1 | |
| 90 | 95.6 | 86.9 | 84.7 | 72.9 | |
| 120 | 99.8 | 96.2 | 93.8 | 85.6 | |
| 150 | 99.8 | 99.7 | 96.4 | 95.8 | |
| 180 | 99.8 | 99.7 | 98.2 | 96.5 | |
| 210 | 99.8 | 99.7 | 98.2 | 96.5 | |
| 240 | 99.8 | 99.7 | 98.2 | 96.5 |
Consequence of Contact Time on Decolorization
The effect of contact time on the removal of AB-43 was evaluated for various concentrations of nano composite (10 to 40 mg/L), at different time interval 30 to 240 minutes depicts an increase in dye removal with increase in time and concentration of the catalyst. The increase in catalyst amount increased the number of active sites on the photocatalyst surface causing increase in the number of •OH radicals which take part in actual decolorization of dye solution. It is clear that the percentage of degradation colour progressively increased 30 to 90 mg/L, thereafter there is no degradation of colour will occur on AB-43 (Fig 7 & 8).
Consequence of Catalyst Dosage on Decolorization
The effect of concentration was analyzed to optimize the amount of nano composite SiO2/TiO2and SiO2/Al2O3 photocatalyst decolorization of AB-43. The experiments were set up with varying the dose from 10 to 40 mg/L for AB-43. The solutions were kept under sunlight illumination for a period of 30 to 240 minutes. The solutions were then centrifuged at 12,000 rpm for 10min and the supernatant was used for water quality analysis by spectrometrically using UV-Visible spectrometer. It can be deduced from the results obtained that the photocatalytic efficiency of SiO2/TiO2 and SiO2/Al2O3 nano-composites. It could be deduced from the results obtained that photocatalytic efficiency of SiO2/TiO2 and SiO2/Al2O3nano composites were shown to be remarkably greater in decolorization of AB-43 dye from 40 mg/L dosage. It is clear that the photocatalytic activity increase with the increase in the dosage of SiO2/TiO2 and SiO2/Al2O3nano composites (Fig 9 & 10).
Decolorization efficiency (%) was calculated by the following formula and all experiments were done in triplicate.
% decolorisation = ((Absorbance at initial –Absorbance at final)/Absorbance at initial) X 100
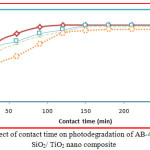 |
Figure 7: Effect of contact time on photodegradation of AB-43 dye using SiO2/ TiO2 nano composite. |
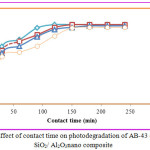 |
Figure 8: Effect of contact time on photodegradation of AB-43 dye using SiO2/ Al2O3nano composite. |
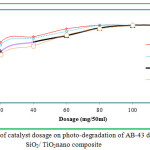 |
Figure 9: Effect of catalyst dosage on photo-degradation of AB-43 dye using SiO2/ TiO2nano composite. |
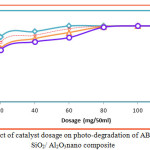 |
Figure 10: Effect of catalyst dosage on photo-degradation of AB-43 dye using SiO2/ Al2O3nano composite. |
Conclusion
SiO2/TiO2 and SiO2/Al2O3 nano-composites were successfully synthesized by sol-gel technique and it was characterized by SEM, TEM and EDAX. The synthesized nano-composites were used for the photodegradation of AB-43 dye. The mixed metal-oxide nano-composites possess greater photodegradation effect of AB-43 under natural sun light. It is clear that the percentage of degradation of colour progressively increased from 30 to 90 percent. The percentage degradation of colour progressively increased with contact time up to 240 minutes. This environmental friendly composite material was used for the degradation of AB-43 dye with irradiation solar light. It can be concluded that nano-composites of SiO2/TiO2 and SiO2/Al2O3 is more efficient photocatalyst on AB-43 dye.
Acknowledgement
This research work a was guided and supported by Dr. Venckatesh Rajendran and Dr. Rajeshwari Sivaraj, Department of Chemistry, Government Arts College, Udumalpet – 642 126.
References
- Dimitrijevic, N. M., Saponjic, Z. V., Rabatic, B. M. and Rajh, T., Assembly and charge transfer in hybrid TiO2 architectures using Biotin−Avidin as a Connector, J.Am. Chem. Soc.,2005. 127, 1344-1345.
CrossRef - Balachandran, K., Venckatesh, R., Rajeshwari Sivaraj, Int. J. Eng. Sci. Technol.,2010.2(8) 3695-3700.
- Bandara, J., Tennakone, K. and Jayatilaka, P. P. B. ,Composite tin and zinc oxide nanocrystalline particles for enhanced charge separation in sensitized degradation ofdyes, Chemosphere,2002.49, 439.
- Singh SK, Singh KN. Eco‐friendly and facile one‐pot multicomponent synthesis of acridinediones in water under microwave. Journal of Heterocyclic Chemistry. 2011. 48(1):69-73.
CrossRef - Wang, Z., Jiangx Zhang, B. Q., Kang, Y., Xie, P. Y., Lv,R., Xu, Zhang, X., Sono catalytic degradation of some dye stuffs and comparison of catalytic activities of nanosized TiO2, nanosized ZnO and composite TiO2 /ZnO powders under ultrasonic irradiation, Ultrasonics, sonochemistry,2009.16, 225-231.
- Xu, L. L., Li, Z. M., Cai, Q. H., Wangn, H. X., Gao, H., Lu, Q., Liu, J., Precursor template synthesis of three dimensional mesoporous ZnO hierarchical structures and their photocatalytic properties, Cryst. Eng. Comm., 2010.12, 2166-2172.
- Xu, X., Tian, J, Wang, X., Dai, J. and Liu, X., Hydrothermal and post-heat treatments of TiO2/ZnO composite powder and its photodegradation behavior on methyl orange, Ceramics International,2011. 37, 2201- 2206.
CrossRef - Yoon, K. H., Cho, J. and Kang, D. H., ibid.1999. 34(9), 1451- 1461.
- Yu, J. G., Zhao, X. J. and Zhao, Q. N., Photocatalytic activity of nanometer TiO2 thin films prepared by the sol–gel method, Mater. Chem. Phys., 2001.69, 25.
- Tian.J.; Chen, L.; Yin, Y.; Wang, X.; Dai, J.; Zhu, Z.; Liu, X; Wu, P. Surface and Coatings Technology,2009. 204(1-2), 205.
CrossRef - Calleja,G., Serrano, D. P., Sanz, R., Pizarro, P. and Garcı´a, A., Study on the synthesis of high-surface-area mesoporous TiO2 in the presence of non-ionic surfactants, Ind. Eng. Chem.Res., 2004.43(10), 2485-2492.
- Diamandeseu, L., Vasiliu, F., Tarabasanu-minaila, D., Feder, M., Vlaicu, A. M., Trodorescu, C. M., Macovei, D., Enculescu, I., Paravulescu,V. and Vasile, E., Structural and photocatalytic properties of iron and europium doped TiO2 nanoparticles obtained under hydrothermal conditions, Mater. Chem. Phy., 2008.112, 146- 153.
- Rajendran Venckatesh, Kartha Balachandaran, and Rajeshwari Sivaraj, 2012,Synthesis and characterization of nano TiO2-SiO2: PVA composite – a novel route, International Nano Letters,2012,2(1),25-29.
CrossRef - Dave, R. S. and Patel, A. R., Photochemical and photocatalytic of cypermethirin under UV radiation, Der.pharma chemical,2010. 2 (1), 152-158.

This work is licensed under a Creative Commons Attribution 4.0 International License.









