Synthesis, Characterization and Herbicidal Activity of Amide Derivatives of Glyphosate
Jatinder Pal Kaur Gill1, Nidhi Sethi2 and Anand Mohan3
and Anand Mohan3
1School of Chemical Engineering and Physical Sciences, Department of Chemistry, Lovely Professional University, Phagwara , Punjab, 144411, India.
2Chemistry Department, Dayanand Mathra Dass College, Moga, Punjab, 142001, India.
3School of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab, 144411, India.
Corresponding Author E-mail: sethinidhi203@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340519
Article Received on : 21-07-2018
Article Accepted on : 12-08-2018
Article Published : 02 Oct 2018
The present work includes synthesis of a series of amide derivatives of glyphosate and their characterization. The structure analysis of these new derivatives was done with the help of FTIR and 1H NMR, Further, their herbicidal activity was analyzed on one of the common weeds (Parthenium hysterophorus). Under the influence of amide derivatives of glyphosate it was found that the chlorophyll content (Chlorophyll a, Chlorophyll b and total Chlorophyll content) of the weed was found to lessen than the control. Moreover, these synthesized derivatives are less polar as compared to the parent glyphosate molecule thereby can emphatically reduce the problem of their leaching into the groundwater.
KEYWORDS:Amide Derivatives of Glyphosate; Glyphosate; Herbicides; Weed
Download this article as:| Copy the following to cite this article: Gill J. P. K, Sethi N, Mohan A. Synthesis, Characterization and Herbicidal Activity of Amide Derivatives of Glyphosate. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Gill J. P. K, Sethi N, Mohan A. Synthesis, Characterization and Herbicidal Activity of Amide Derivatives of Glyphosate. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50104 |
Introduction
Agrochemicals have become global necessity to increase crop productivity in agricultural fields.1-5 Nowadays they play a pivotal role in controlling not only the pests and rodents but also many microbial infections.5-7 There are several types of herbicides, insecticides and pesticides that are in use in the modern cultivation of lands.7-12
The invention of glyphosate (N-(phosphonomethyl) glycine) was a big breakthrough in that era. Ever since its discovery in the year 1970, it is still the most commonly used herbicide all over the world.13 Glyphosate [N-(phosphonomethyl) glycine] is a non-selective, systemic, foliar applied, post emergent herbicide which is widely used to control the weeds.14 Glyphosate is the only herbicide that kills the plant by inhibiting the synthesis of enzyme 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPS). EPSPS is a vital enzyme of Shikimate pathway needed for the bio synthesis of aromatic amino acids (phenylalanine, tyrosine, and tryptophan).15 Due to large-scale use of glyphosate in the farming, glyphosate-resistant crops were introduced in the market in 1996. Glyphosate-resistant soybeans, cotton and corn crops are cultivated all over the world16. Later on with the advancements in biotechnology, CP4 gene of Agrobacterium sp. was used to encode glyphosate-resistant 5-enolpyruvyl-shikimate-3-phosphate synthase. These CP4 genes along with some promoters were introduced into the genome of various crops which show high levels of glyphosate resistance.17 Due to excessive use of glyphosate in the agricultural lands several damaging and toxicological effects have been shown on non-target organisms found in water and soil.18
To surmount the problems caused by excessive use of glyphosate, certain structural modifications were done on the glyphosate to improve its properties. A series of amide derivatives of glyphosate was successfully synthesized to improve the properties of glyphosate and to solve the problem of weed resistivity. A series of amide derivatives of glyphosate were synthesized by condensation of glyphosate with required amine separately using mixed anhydride method of coupling with Isobutyl chloroformate as a coupling reagent in presence of inert solvent with slight modifications.19-20 Herbicidal activity of these derivatives have also been checked on the weed (Parthenium hysterophorus).
Material and Methods
Reagents
Glyphosate (Technical Grade 95.10%), N-Methylmorpholine (AR), Isobutyl chloroformate (AR), Methanamine, Propan-2-amine, Butan-1-amine, Solvents; Dioxane (AR), Ethyl acetate (AR)
Synthesis of Amide Derivatives of Glyphosate
Glyphosate (10mm,1.69g) was dissolved in 10mL of dioxane ( minimum amount of NaOH pellets were dissolved in it) under ice-cold conditions (-5°C) at constant stirring. To that N-Methylmorpholine (13mm,1.42mL) was added followed by addition of Isobutyl chloroformate (13mm,1.68mL) as a coupling reagent. Then the corresponding amine (1.3equiv.) was added to the reaction mixture. The contents of the flask were stirred for 2 hours. The solvent (dioxane) was evaporated under reduced pressure and the residue obtained was extracted by using ethyl acetate followed by washings with cold 5% aqueous citric acid, brine, 5% aqueous NaHCO3 and finally with brine. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude product was purified over silica gel column using ethyl acetate and hexane and was dried in vaccum dessicator using P2O5.
Characterization was done using FTIR and 1H NMR. The IR spectra were recorded by Schimadzu spectrophotometer, 1H NMR spectra were recorded in D2O with TMS as internal standard using Bruker Advance II instrument at 400MHz.
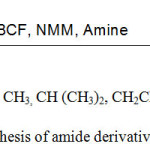 |
Scheme 1: Synthesis of amide derivatives of glyphosate. |
Herbicidal Activity of Synthesized Derivatives of Glyphosate
As glyphosate is a post-emergence herbicide, accordingly the post-emergent herbicidal effects of synthesized derivatives of glyphosate were checked against the common weed Parthenium hysterophorus.21 Determination of the Chlorophyll content (Chlorophyll a, chlorophyll b and chlorophyll a+b) was used as an important parameter to evaluate the detrimental and herbicidal effects of these synthesized derivatives on it.22 Synthesized derivatives had shown moderate to good herbicidal properties at all the test concentrations in comparison to control.
Seeds of Parthenium hysterophorus were collected from wild population naturally grown around wild areas of Kapurthala, Punjab. The viability of seeds was checked in the laboratory, where the seeds were set to germinate in pre-sterilized Petri plates and exhibited 90 % germination. Poly bags were filled with 2 kg sandy loam soil. Ten seeds of Parthenium hysterophorus were sown in each pot and were watered regularly at field capacity. Two weeks after sowing, when maximum germination had been attained, the numbers of plants were reduced to have a uniform density. Three different concentrations of each derivative and the herbicide glyphosate (as negative control) were selected1 X (recommended dose 441g/L per acre), 0.5 X (half of recommended dose) and 0.25 X (one-fourth of recommended dose)]. Each treatment was replicated three times. The required amount of each compound was applied to all bags except the control (Distilled water). A simple hand sprayer was used to apply the compounds on the plants. The plants foliage was sprayed sufficiently so that it is completely wet with the compounds. The experiment was carefully watched on daily basis for the development of any symptoms (necrosis, wilting and complete death of plant). Leaf samples were collected for the determination of chlorophyll a, chlorophyll b and total chlorophyll content.
Results
Synthesis of Amide Derivatives
(a) ({[2-(methylamino)-2-oxoethyl]amino}methyl)phosphonic acid:
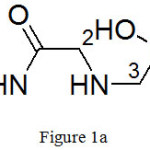 |
Figure 1a |
Molecular formula: C4H11N2O4P, yield: 23%, Brown gummy solid, FTIR (KBr cm-1)-8400S: 3620 (-NH stretching), 2966 (-CH asym stretching), 2871( -CH sym stretching), 2362, 1736 (O=P-OH), 1655 (C=O stretching), 1514 (-NH bending), 1462 (C-H asym bending), 1281 (P=O stretching), 1127 (-CN stretching), 1073,971(P-O stretching), 742, 668 (P-C stretching) 1H NMR (400 MHz, D2O): (Fig. 4(a)) 0.556 (2H, s, H-3), 2.54 (3H, s, H-1), 3.69 (2H, s, H-2)
(b) ({[2-oxo-2-(propan-2-ylamino)ethyl]amino}methyl)phosphonic acid:
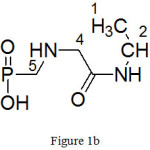 |
Figure 1b |
Molecular formula: C6H15N2O4P, yield: 33%, Colourless liquid
FTIR (KBr cm-1)-8400S: 3327 (-OH stretching), 3056 (-NH stretching), 2969 (-CH asym stretching), 2875(-CH sym stretching), 2362 ( O=P-OH), 1695 (C=O stretching), 1536 (-NH bending), 1469 (C-H asym bending), 1385 [CH2 deformation (gem-dimethyl)], 1250 (P=O stretching), 1187 (-CN stretching), 1086,980 (P-O stretching), 779,645 (P-C stretching).1H NMR (400 MHz, D2O): (Fig. 4(b)) 0.82-0.86 (6H, dd, J=1Hz, H-1, 3), 1.06 (2H, s, H-5), 1.81 (2H, s, H-4), 3.83-3.86 (1H, m, H-2)
(c) ({[2-(butylamino)-2-oxoethyl]amino}methyl)phosphonic acid:
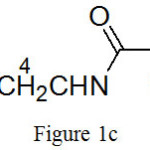 |
Figure 1c |
Molecular formula: C7H17N2O4P, yield: 48%, Colourless liquid, FTIR (KBr cm-1)-8400S: 3334 (-OH stretching), 3068 (-NH stretching), 2959 (-CH asym stretching), 2874(-CH sym stretching), 2340 ( O=P-OH), 1698 (C=O stretching), 1537(-NH bending), 1468 (C-H asym bending, 1250 (P=O stretching), 1141 (-CN stretching)1054,966 (P-O stretching),739,639 (P-C stretching).1H NMR (400 MHz, D2O): (Fig. 4(c)) 0.78- 0.86 (3H, m, H-1), 1.16-1.28 (2H, m, H-2), 1.32-1.41 (2H, m, H-3), 2.53 (2H, s, H-6), 2.98- 3.04 (2H, m, H-4), 3.54-3.56 (2H,d, J=7.2 Hz, H-5)
Herbicidal Activity of Amide Derivatives of Glyphosate
Amide derivatives of glyphosate have shown the herbicidal effects on the weed Parthenium hysterophorus. Significant reduction in the photosynthetic pigments (chlorophyll a, chlorophyll b and chlorophyll a+b ) have been observed in all cases with respect to time and concentration as compared to normal control (Table 1). But this decrease was not comparable to glyphosate. Out of the three different synthesized amide derivatives, Isopropyl amide derivative of glyphosate [({[2-oxo-2-(propan-2-ylamino)ethyl] amino}methyl) phosphonic acid] is more effective in decreasing the chlorophyll a, chlorophyll b and chlorophyll a+b content in the plant at all the three concentrations. At 0.25X concentration, an effective decrease was observed in chlorophyll a (0.292µg/gFW) chlorophyll b(0.181µg/gFW) and chlorophyll a+b (0.441 µg/gFW). However with increase in concentration of isopropyl amide derivative i.e at 1X concentration the further noticeable drop off in the chlorophyll pigments have been noticed [chlorophyll a (0.242 µg/gFW); chlorophyll b (0.145 µg/gFW); chlorophyll a+b (0.407 µg/gFW)].
 |
Table 1: Effect of synthesized amide derivatives of glyphosate on the chlorophyll content a (µg/gFW), chlorophyll content b(µg/gFW) and chlorophyll a+b Content (µg/Gfw) In The Leaves Of Parthenium Hysterophorus. |
Where 0.25X= One fourth of the recommended dose, 0.5X= Half of recommended dose and 1X= Recommended dose (441g/L per acre) .Values are mean of ± SD (n=3).
Other two amide derivatives also follow the same trend of reduction in chlorophyll content ( chlorophyll a, chlorophyll b and chlorophyll a+b) with increase in concentration of the derivative with respect to time. After 15 days of application, a momentous change in the amount of photosynthetic pigments have been observed in comparison to control. In case of methyl amide of glyphosate [({[2-oxo-2-(propan-2-ylamino)ethyl]amino}methyl)phosphonic acid] and butyl amide of glyphosate [({[2-(butylamino)-2-oxoethyl]amino}methyl)phosphonic acid] at 0.25X concentration chlorophyll a found was 0.331µg/gFW and 0.311µg/gFW respectively. While at 1X, chlorophyll a get reduced to 0.274 µg/gFW and 0.260 µg/gFW respectively. Similarly amount of chlorophyll b left at 0.25X (after 15 days) was 0.152 µg/gFW in case of methyl amide and 0.173 µg/gFW in case of butyl amide of glyphosate. This reduction is more manifested at higher concentation of 1X.
Along with chlorophyll content determination, a significant increase in the number of dead plants was observed with exposure of Parthenium hysterophorus to the synthesized amide derivatives of glyphosate. This increase was manifested with increase in the concentration of the derivative and time. At 0.25X, in case of methyl amide of glyphosate only 1 plant was dead even after 15 days. At 0.5X, not much change was noticed even after 15 days of treatment. But at 1X, half of the plant population (5 out of 10) was dead. Isopropyl amide of glyphosate also followed the similar trend at all the three test concentrations (Figure4(d)). However butyl amide of glyphosate killed the minimum number of plants (only 2 after 15 days at 1X) and does not show much herbicidal activity.
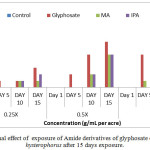 |
Figure 4d: Lethal effect of exposure of Amide derivatives of glyphosate on Parthenium hysterophorus after 15 days exposure. |
Values are mean of 3 (n=3)
Where 0.25X = One fourth of recommended dose, 0.5X= Half of recommended dose and 1X= Recommended dose).
MA = ({[2-(methylamino)-2-oxoethyl]amino}methyl)phosphonic acid; IPA = ({[2-oxo-2-(propan-2-ylamino)ethyl]amino}methyl)phosphonic acid ;BA = ({[2-(butylamino)-2-oxoethyl]amino}methyl)phosphonic acid.
Conclusion
Current work has successfully conducted an efficient synthesis of amide derivatives of glyphosate using mixed anhydride method. The method used was highly effective to obtain very good results even with a simple synthetic procedure. It also resulted in an easy product separation and high purity of products. The results showed that all these derivatives have decreased the chlorophyll content of the sample weed and finally killed the plant. In particular, Isopropyl amide derivative of glyphosate [({[2-oxo-2-(propan-2-ylamino)ethyl]amino}methyl) phosphonic acid] is more effective in decreasing the chlorophyll a, chlorophyll b and chlorophyll a+b content in the plant at all the three concentrations. Thus, we can definitely conclude that these newly synthesized derivatives of glyphosate are good herbicides and can be used as a substitute of the glyphosate.
Acknowledgements
We acknowledge the SAIF department of Panjab University, Chandigarh for providing the highly sophisticated laboratory facilities for carrying out characterization work.
References
- Kumar, V.; Kaur, S.; Singh, S.; & Upadhyay, N. 3 Biotech, 2016, 6(1), 1.
- Kumar, V.; Singh, S.; Singh, J.; Upadhyay, N.;. Bull. Environ. Contam. Toxicol. 2015b 94, 807-815.
CrossRef - Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A. B.; Singh, D.; & Singh, J. Environ. Chem. Letters, 2016, 14, 317-329.
CrossRef - Singh, S.; Kumar, V.; Upadhyay, N.; Singh, J.; Singla, S.; Datta, S.; 3 Biotech, 2017a 7(4):262.
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A. B.; Singh, N.; & Singh, J. Environ. Chem. Letters, 2017, 1-27.
- Kumar, V.; Singh, S.; Singh, R.; Upadhyay, N.; & Singh, J J. Chem. Biol, 2017, 10(4), 179-190.
CrossRef - Kumar, V.; Upadhyay, N.; Kumar, V.; Kaur, S.; Singh, J.; Singh, S.; & Datta, S. . J Biodivers Environ Sci, 2014, 5, 111-120.
- Kumar, V.; Upadhyay, N.; Singh, S.; Singh, J.; & Kaur, P. Curr World Environ, 2013, 8(3), 469-472.
CrossRef - Kumar, V.; Singh, S.; Manhas, A.; Singh, J.; Singla, S.; & Kaur, P. Oriental J Chem, 2014, 30(4), 1771-1776.
- Mishra, V.; Gupta, A.; Kaur, P.; Singh, S.; Singh, N.; Gehlot, P.; & Singh, J.. Int J phyt, 2016, 18(7), 697-703.
- Kumar, V.;Singh, S.; Kashyap, N.; Singla, S.; Bhadrecha, P.; & Kaur, P. Orient. J. Chem, 2015, 31(1), 357-361.
CrossRef - Kaur, P.; Singh, S.; Kumar, V.; Singh, N.; & Singh, J. Int. J. Phyt. 2017.
- Duke, S.O.; Powles, S.B. Pest. Manag Sci. 2008, 64(4), 319–325
CrossRef - Benbrook, C. M. Environmental Sciences Europe. 2016, 28, 3 https://doi.org/10.1186/s12302-016-0070-0.
CrossRef - Johnson, W.G,; Davis, V.M,; Kruger, G.R,; Weller, S.C. Eur. J. Agron. 2009, 31(3),162–172
CrossRef - Duke, S.O,; Lydon, J,; Koskinen, W.C,; Moorman, T.B,; Channey, R.L,;Hammerschmidt, R. J. Agric. Food. Chem. 2012, 60(42), 10375–10397
CrossRef - Padgette, S.R,; Re, D.B,; Barry, G.F,; Eichholtz, D.E,; Delannay, X,; Fuchs, R.L,; Fraley, R.T. Herbicide resistant Crops. 2018, 65-100
- Alliance, G. T,; Glyphosphate Fact Sheet. Pesticides News. 1996, 33, 28-29. http://www.forumforpages.com/facebook/gmo-truth-alliance/glyphosphate-fact-sheet/4261401401/0
- Vaughan, Jr, J. R. J. Am. Chem. Soc. 1951, 73(7), 3547-3547
CrossRef - Chen, F. M.; Benoiton, N. L. Can. J. Chem. 1987, 65(3), 619-625
CrossRef - Shabbir, A. Pak. J. Weed. Sci. Res. 2014, 20, 1-10.
- Lichtenthaler, H. K. Methods. Enzymol. 1987, 148, 350-382
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









