Synthesis and Evaluation of Antimicrobial Activity of New Imides and Schiff Bases Derived from Ethyl -4-Amino Benzoate
Wurood S. Ahmed1, Ammar A. Razzak Mahmood2 and Redha I. Al-Bayati3
and Redha I. Al-Bayati3
1 Al-Yarmouk University, Pharmacy Department, Baghdad-Iraq.
2 Pharmaceutical Chemistry Department, College of Pharmacy-University of Baghdad-Baghdad,10001,Iraq.
3Chemistry Department, College of Science, Al-Mustansirya University, Baghdad, 10001,Iraq.
*Corresponding Author E-mail: kubbaammar1963@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340533
Article Received on : 06-08-2018
Article Accepted on : 29-09-2018
Article Published : 19 Oct 2018
A series of disubstituted 1,3,4-oxadiazole derivatives, including imides and Schiff bases, was achieved from the starting material, ethyl-4-aminobenzoate, which was converted to the corresponding 4-aminobenzohydrazide (1), by its reaction with hydrazine hydrate in absolute ethanol. Two oxadiazole parent nuclei had been synthesized from (1), the first nucleus 5-(4-aminophenyl)-1,3,4-oxadiazol-2-amine(2), and the second is 5-(4-aminophenyl)-1,3,4-oxadiazole-2-thione (3). Compound (2) Obtained from stirring methanolic solution of (1) with cyanogen bromide (CNBr) and sodium bicarbonate (NaHCO3) at RT. While compound (3) was synthesized by refluxing of (1) with CS2 in the presence of (KOH), the produced potassium salt of hydrazide underwent cyclization by acidification with 10% HCl. Meanwhile, the cyclic imides derivatives (4-6) and (10-12) were synthesized by thermal fusion of (2) or (3) with acid anhydrides, While Schiffʼs bases derivatives (7-9) and (13-15) were synthesized by a conventional method involved refluxing of (2) or (3) with different aromatic aldehydes, in acidic medium (using glacial acetic acid). The new derivatives had been tested against three Gram-positive bacteria (Staphylococcus aureus, Micrococcus luteus, and Bacillus pumilus), and two Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli),and two fungal species: (Saccharomyces cerevisiae and Candida albicans). Among the synthesized derivatives, compound (15) displayed a moderate to potent antibacterial activity, against different (Gram- positive and Gram- negative) bacteria, and also showed a slight to moderate antifungal activity.
KEYWORDS:Antimicrobial; Imides; Schiff base; Synthesis; 1,3,4-Oxadiazole derivatives
Download this article as:| Copy the following to cite this article: Ahmed W. S, Mahmood A. A. R. M Bayati R. I. A. Synthesis and Evaluation of Antimicrobial Activity of New Imides and Schiff Bases Derived from Ethyl -4-Amino Benzoate. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Ahmed W. S, Mahmood A. A. R. M Bayati R. I. A. Synthesis and Evaluation of Antimicrobial Activity of New Imides and Schiff Bases Derived from Ethyl -4-Amino Benzoate. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=51157 |
Introduction
1,3,4-Oxadiazole is a heterocyclic five –member ring possessing one oxygen atom and two nitrogen atoms. It is originated from a furan ring ,where two methylene groups (=CH) substituted with two pyridine type nitrogen atoms (-N=).1,2 The 1,3,4-oxadiazole nucleus and their 2,5-disubstituted derivatives can go significant pharmacological activity including, anti inflammatory,3,4 anticancer,5,6 antibacterial,7 antifungal,8anti-HIV 9 and antioxidant activities 10
1,3,4-oxadiazole derivatives havethe ability to go through different chemical reactions , which made them essential for new molecule preparation, due to their distinct chemical structure, that has vast pharmacological importance.11
Many methods in the literature document the synthesis of 1,3,4-oxadiazoles,1,12,13 The more commonly used pathway for 1,3,4-oxadiazoles preparation, includes reactions of acid hydrazides with carboxylic acids / acid chlorides then after, cyclization or ring closure of diacylhydrazines via some of dehydrating agents ,for example phosphorous oxychloride.14-18
It has been documented that Gram- negative bacteria are much more resistant to different antimicrobial agents, as compared to Gram-positive bacteria.19 The differences may be due to, the constituent of the cell wall in Gram- positive bacteria is of a simple and single layer, while the cell wall in Gram-negative bacteria possesses an “outer membrane” with high lipid and lipoprotein content, which is not found in Gram- positive bacteria.20 Therefore the lipophilic nature of the cell membrane of Gram-negative bacteria is more resistant towards antibacterial agents, which functions as a strong barrier for a variety of antimicrobial agents. Therefore, compounds possessing hydrophilic properties, will not be able to penetrate the cell membranes of Gram-negative bacteria. Meanwhile, the cell wall of Gram- positive bacteria is not a complex structure like Gram- negative bacteria. Antibacterial agents can quickly collapse the bacterial cell wall of Gram-positive bacteria, which leads to disruption of the cytoplasm.21
This work targets to synthesize of new derivatives of cyclic imides and Schiff bases derived from two parent nuclei: 5-(4-aminophenyl)-1,3,4-oxadiazol-2-amine (2), and 5-(4-aminophenyl)-1,3,4-oxadiazole-2-thione (3), respectively. Imides are essential to lead components for natural products and drugs.22 While, Schiff bases have been widely studied and the focus of different researches, due to their extensive use and distinct biological activities.23
Materials and Methods
The starting material for the synthesis of 1,3,4-oxadiazole derivatives is ethyl-4-amino benzoate, and the first step of the reaction involves the formation of the corresponding hydrazide then after, preparation of two- parent nuclei (1) and (2).
All chemicals and solvents used during synthesis were of AR grade and used without further purification. Reaction monitoring was ascertained by thin-layer chromatography (TLC), using Silica gel GF254 (type 60) pre-coated Aluminium sheets, Merck (Germany) exposed to UV-254 nm light, and the eluent used for TLC as follows: ethyl acetate: n-hexane 9:1 (for compounds (1),(4) (5), (6) ,(8),(9),(11)and (12); ethyl acetate: n-hexane 9.5:0.5. for compound (2); ethyl acetate: n-hexane 6:4, for compounds (3), (10) ,(14)and (15); ethyl acetate: n-hexane 5:5, for compounds (7) and (13). All synthesized derivatives were characterized by spectroscopic analysis (FTIR and 1HNMR).
Melting points were measured using melting point apparatus in open capillary tubes, and are uncorrected. The IR spectra, were recorded, on( FTIR-600,UK) spectrophotometer, using (KBr disc), (ύ,cm-1).
Furthermore, 1HNMR spectra were recorded on Bruker model Ultra shield 300 MHz, Avance II- at (Al-Bayt University-Jordan), using tetramethylsilane (TMS) as an internal standard, the chemical shift was expressed as (δ=ppm), DMSO-d6 and acetone-d6 were used as solvents.
Chemical Synthesis
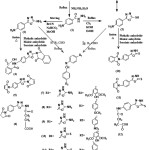
Chemical synthesis of all new derivatives is depicted in (scheme 1).
Synthesis of 4-aminobenzohydrazide (1)24
Ethyl-4-aminobenzoate (0.06mol, 9.9g) was dissolved in (30 mL) of hydrazine hydrate 80%, then absolute ethanol (25 mL) was added in a rounded bottom flask and refluxed for 8 h. At the end of the reaction, as monitored by TLC, the mixture was cooled to room temperature (RT), the crystals formed, filtered and recrystallized from absolute EtOH.
White crystals, yield 77%, m.p 222-225°C, IR(KBr), ( υ, cm-1): 3271 and 3234 prim. (NH2) str, 3033 Ar(CH) str,1626 (C=O) amide str, 1604 (NH) bend, 1545 (C=C) str, 843 out of plain (C-Hbenz.) bend.
Synthesis of 5-(4-aminophenyl)-1,3,4-oxadiazol-2-amine (2)25
4-Aminobenzohydrazide (1), (0.013mol, 2.0g) was dissolved in (40 mL) of MeOH with a solution of NaHCO3 (0.013mol, 1.0 g) in (20 mL) of distilled water, then addition of cyanogen bromide (CNBr), (0.013mol,1.4g), and stirring the mixture in a round flask overnight at RT, then methanol was evaporated, and (30mL) of cold distilled water was added, the precipitate formed, obtained by filtration. Off-white to pink powder, yield 61%, m.p 280-282 ᵒC, IR(KBr), (υ, cm-1): 3327 & 3224 prim (NH2) str, 3060 (Ar-CH)str, 1608 (C=N) str, 1585,1570&1506 (C=C) str,1290 asym (C-O-C) str, 1068 sym (C-O-C) str, 837 out of plain(C-Hbenz.) bend; 1HNMR(300 MHz,DMSO-d6,δ= ppm): 7.40 (s,2H,NH2); 6.85-6.50 (m,4H, 4Ar-H); 5.60 (s,2H, NH2-oxadiaz).
Synthesis of 5-(4-aminophenyl)-1,3,4-oxadiazole-2-thione (3 )24
4-Aminobenzohydrazide (1) (0.02 mol, 3.0 g) dissolved in absolute EtOH (20 mL) and cooled to 20°C in the ice bath. Then after, addition of potassium hydroxide (KOH) to the mixture,(0.027 mol,1.5 g) in an absolute EtOH (10 mL), and stirred for 15 min. Then CS2 (0.024 mol, 1.5 mL) was added gradually. The reaction was refluxed for 12 h, The solvent was reduced by a rotary evaporator, and the produced solid was dissolved in (25 mL) of cold D.W and acidified with 10% HCl, until precipitation of light yellow crystals of (3) obtained and recrystallized from absolute EtOH.
Light yellow crystals, yield 64%, m.p 243-245ᵒC, IR(KBr), (υ, cm-1): 3315 and 3220 prim (NH2) str, 3059 Ar(CH) str, 2557 str of (SH) thiol gr,1618 (NH) bend, 1603 cm-1 (C=N) str, 1570, 1512 and 1485 (C=C)str,1271 asym (C-O-C) str, 1065 sym (C-O-C) str, 829 (out of plain C-Hbenz.)bend; 1HNMR (300 MHz, DMSO–d6,δ=ppm): 7.48 (d,2H, 2Ar-H); 6.60 (d,2H, 2Ar-H); 5.90 (brs, 1H,SH); 7.78 (s,2H, NH2).
General method for the synthesis of imide derivatives (4-6)
In a pyrex test tube, added compound (2) (0.0011mol, 0.19g), and phthalic anhydride (0.0022mol, 0.325g) or maleic anhydride(0.0022mol, 0.215g), or succinic anhydride (0.0022mol, 0.22g), mixed and heated using oil bath 290°C. The mixture stirred continuously by using pyrex glass road for 30 min, then washed with diethyl ether and left to dry.
Synthesis2-((4-(5-(1,3-dioxoisoindolin-2-yl)-1,3,4-oxadiazol-2-yl)phenyl)carbamoyl)benzoic acid (4)
Light yellow powder, yield 95%, m.p 286-288°C, IR(KBr), (υ, cm–1):3307 (OH of COOH) str,3095 Ar(CH) str, 1749 (C=O) acid str, 1685 and 1651 asym. & sym (C=O) amide str, 1604 (C=N) str, 1557 (C=C) str, 1257 asym (C-O-C) str, 1043sym (C-O-C) str, 835 out of plain (C-H benz.) bend;1 HNMR(300 MHz, DMSO–d6,δ=ppm): 8.18-7.18 (m,12H, 12Ar-H); 10.60 (s,1H, OH).
Synthesis of 1-(4-(5-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-1,3,4-oxadiazol-2-yl)phenyl)-1H-pyrrole-2,5-dione (5)
Yellow powder, yield 92%, m.p 244-246ᵒC, IR(KBr), (υ,cm-1): 3012 Ar(CH) str,1664 (C=O) amide str,1604 (C=N) str, 1552,1510 and 1427 (C=C) str,1238 asym (C-O-C) str, 845 out of plain (C-H benz.) bend; 1HNMR (300MHz, DMSO–d6,δ=ppm): 7.72 (m,4H,4Ar-H); 7.12 (d,2H, maleimide -2H); 6.36(d,2H, maleimide-2H);10.55 (s,1H,OH (keto/enol form).
Synthesis of 4-((5-(4-(2,5-dioxopyrrolidin-1-yl)phenyl)-1,3,4-oxadiazol-2-yl)amino)-4-oxobutanoic acid (6)
White powder, yield 89%, m.p 252-254ᵒC, IR(KBr), (υ, cm-1) : 3315 (OH) str, 2935 asym (CH2), 1725 (C=O) acid str,1701 and 1666 (C=O amide) str, 1606 (C=N) str,1583 and 1506 (C=C) str, 1061 sym (C-O-C) str, 835 out of plain (C-H benz.) bend; 1HNMR (300MHz, DMSO–d6,δ= ppm: 12,10 (s,1H,OH);10.16 (s,1H,NH); 7.93-7.08 (m,4H,4Ar-H); 2.76 (t,2H,CH2).Other CH2 &2CH2 groups of succinimide, were masked with DMSO-d6 peak.
Synthesis of (E)-4-(((4-(5-amino-1,3,4-oxadiazol-2-yl)phenyl)imino)methyl)-2,6-dimethoxyphenol (7)
Glacial acetic acid (3dr) was added to 3,5-dimethoxy -4-hydroxy benzaldehyde (0.0036mol, 0.65g ), dissolved in (20 mL) of MeOH, the entire mixture stirred in a round bottom flask for 15 min, then compound (2), (0.0017mol, 0.3g ) in (20 mL) of MeOH was added, and refluxed for 5 h. At the end of the reaction, the mixture was cooled, then poured into crushed ice to get a precipitate, which was collected by filtration, dried and washed with ethyl acetate, and recrystallized from EtOH.
Yellow powder, yield 53%, m.p138-140ᵒC, IR(KBr), (υ, cm-1): 3573 (OH) str, 3361 and 3329 (NH2) str, 2941 and 2841 asym & sym (CH) str,1606 and 1579 (C=N) str, 1512 and 1462 (C=C) str, 1252 (Ar-O-CH3) str, 1038 sym (C-O-C) str, 839 (out of plain C-H benz.) bend; 1HNMR (300 MHz, DMSO-d6,δ=ppm): 9.77 (s,1H,OH); 8.50 (s,1H,CH=N); 8.17-7.24 (m,6H,6Ar-H); 7.80 (s,2H,NH2 ); 3.84 (s,6H,2×OCH3).
Synthesis of (E)-5-(4-((4-methoxybenzylidene)amino)phenyl)-1,3,4-oxadiazol-2-amine (8)
Glacial acetic acid (3 dr) was added to 4-methoxy benzaldehyde (0.0036 mol, 0.5g), the mixture stirred in a round bottom flask for 15 min, then compound (2), (0.0017mol, 0.3g) in (30 mL) of MeOH was added, and refluxed for 6 h. After completion of the reaction, the mixture was cooled to RT, and poured into crushed ice, to produce a precipitate, then filtered, dried and recrystallized from EtOH.
Yellow powder, yield 55%, m.p 254-256ᵒC, IR(KBr), (υ, cm-1) :3303 and 3240 prim (NH2) str, 3120 (NH-tautomer) str, 2964 & 2839 asym& sym (CH) of CH3 str, 1653 and 1597 (C=N) str, 1570 and 1510 (C=C) str, 1254 (Ar-O-CH3) str, 845 (out of plain C-H benz.)bend; 1HNMR(300 MHz, DMSO-d6, δ=ppm: 8.59 (s,1H,CH=N); 7.93-7.08 (m,8H, 8Ar-H and NH2); 3.85 (s, 3H, 1XOCH3).
Synthesis of (E)-5-(4-((4-nitrobenzylidene)amino)phenyl)-1,3,4-oxadiazol-2-amine (9)
Glacial acetic acid (3dr) was added to 4-nitrobenzaldehyde (0.0036mol, 0.54 g) in (10 mL) of absolute EtOH. The entire mixture stirred in a round bottom flask for 15 min, then compound (2),(0.0017mol, 0.3g) dissolved in (20 mL) of hot absolute EtOH was added, stirred and refluxed for 5 h. The precipitate which was obtained, filtered and recrystallized from EtOH.
Yellow powder, yield 58%, m.p 275-277 ᵒC, IR(KBr), (υ, cm-1): 3317 and 3252 prim (NH2) str, 3080 Ar(CH) str, 2898 (CH) str ,1676 and 1597 (C=N) str, 1516 and 1344 asym &sym (NO2) str, 865 out of plain (C-H benz.) bend; 1HNMR (300 MHz, DMSO-d6, δ=ppm): 8.82 (s,1H,NH); 8.42 (s,1H,CH=N); 8.24 (d,2H,2Ar-H); 7.98 (d,2H,2Ar-H); 7.76 (d, 2H, 2Ar-H); 7.28 (d, 2H, 2Ar-H); 7.50 (s,2H,NH2).
General method for the synthesis of imide derivatives (10-12)
In a pyrex test tube added compound (3),(0.001mol, 0.19g) and phthalic anhydride (0.001mol, 0.148g), or maleic anhydride (0.001mol, 0.1g), or succinic anhydride (0.001mol, 0.1g), mixed and heated using an oil bath 290ᵒC. The mixture stirred continuously by using pyrex glass road for 30 min, then washed with diethyl ether and left to dry.
Synthesis of 2-(4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)isoindoline-1,3-dione (10)
Off-white powder, yield 89%, m.p 289-291ᵒC, IR(KBr), (υ, cm-1): 3225 (NH) str, 3078 Ar(CH) str,1739 and1712 asym & sym (C=O) amide str, 1612 (C=N) str, 1514 and 1469 (C=C) str, 841 out of plain (C-H benz.) bend; 1HNMR(300MHz, DMSO-d6, δ=ppm): 8.06-7.69 (m, 8H,8Ar-H).
Synthesis of 1-(4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)-1H-pyrrole-2,5-dione (11)
Dark yellow powder, yield 95%, m.p 229-231ᵒC, IR(KBr), (υ, cm-1): 3168 (NH) str, 3030 Ar(CH) str,1687 (C=O)str,1626 (NH) bend ,1604 (C=N) str,1514 (C=C) str , 847 out of plain (C-H benz.) bend; 1HNMR(300 MHz, DMSO-d6,δ=ppm): 10.68 (s,1H,NH); 7.87-7.81(m, 4H, 4Ar-H); 6.51 (d,1H, maleimide-H); 6.33 (d,1H, maleimide-H).
Synthesis of 4-oxo-4-((4-(5-thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenyl)amino)butanoic acid (12)
White powder, yield 85%, m.p 233-235ᵒC, IR(KBr), (υ, cm-1) : 3450 ( OH)str, 3323and 3126 (NH) amide str, 3099 Ar(CH) str, 2966 and 2890 asym & sym (CH) of CH2 str, 2652 (SH)str, 1697 (C=O) of COOHstr,1676 (C=O) amide str, 1608 (C=N) str, 1585 ,1516&1493 (C=C) str, 847 out of plain( C-H benz.) bend;1HNMR(300MHz,DMSO–d6,δ=ppm): 14.60(s,1H,OH); 12.10 (s,1H,NH); 10.28(s,1H,NH); 7.75(m,4H,4Ar-H); 3.24(t, 2H,CH2). Other (CH2) gr, masked with DMSO-d6 peak.
Synthesis of (E)-5-(4-((4-hydroxy-3,5- dimethoxy benzylidene)amino)phenyl)-1,3,4-oxadiazole-2(3H)-thione (13)
Glacial acetic acid (3dr) was added to 3,5-dimethoxy -4-hydroxy benzaldehyde (0.0017mol, 0.3g) dissolved in absolute EtOH (20 mL), the mixture stirred in a round bottom flask for 15 min, then compound (3),(0.0016mol, 0.3g) dissolved in hot absolute EtOH (20 mL), was added, and the mixture refluxed for 6 h. After completion of the reaction. It was cooled to RT, a precipitate formed, which was filtered and washed with ethyl acetate, then recrystallized from petroleum ether.
Orange powder, yield 62%, m.p 258-260 ᵒC, IR(KBr), (υ, cm-1): 3519 (OH) str, 3041 Ar(CH) str, 2945 & 2814 asym & sym (CH) of CH3 str, 2569 (SH) str,1651 and 1597 (C=N) str, 1512,1489&1437 (C=C) str,1234 asym (C-O-C)str,1097 sym(C-O-C) str, 862 (out of plain CH benz.) bend; 1HNMR(300MHz,DMSO-d6,δ=ppm): 9.73 (s,1H,OH); 8.84 (s,1H,NH); 8.45 (s,1H,CH=N); 8.03-6.63 (m,6H,6Ar-H); (2×OCH3) masked behind H2O(DMSO–d6).
Synthesis of (E)-5-(4-((4-methoxybenzylidene)amino)phenyl)-1,3,4-oxadiazole-2(3H)-thione (14)
Glacial acetic acid (3dr) was added to 4-methoxybenzaldehyde (0.0017mol, 0.23g ), the mixture stirred in a round bottom flask for 15 min, then compound (3), (0.0016mol, 0.3g) dissolved in hot absolute EtOH(30 mL), was added, and the mixture refluxed for 6 h. At the end of the reaction, it was cooled to RT, and a precipitate formed which was filtered and recrystallized from petroleum ether.
Orange powder, yield 58%, m.p 210-212ᵒC, IR(KBr), (υ, cm-1): 3221 (NH) str, 2931 and 2835 asym & sym (CH) of CH3 str, 1610 (C=N) str,1514and 1406 (C=C) str,1252 asym (C-O-C) str,1070 sym (C-O-C) str, 831 cm-1(out of plain CH benz.)bend;1HNMR(300MHz,DMSO–d6,δ=ppm): 9.83 (s,1H,NH); 8.54 (s1H,CH=N); 7.94-6.53 (m,8H,8Ar-H); 3.81(s, 3H,1×OCH3 ).
Synthesis of (E)-5-(4-((4-nitrobenzylidene)amino)phenyl)-1,3,4-oxadiazole-2(3H)-thione (15)
Glacial acetic acid(3 dr) was added to 4-nitrobenzaldehyde (0.0017mol, 0.26g ), the mixture stirred in a round bottom flask for 15 min, then (3), (0.0016mol, 0.3g) dissolved in hot absolute EtOH (30 mL), was added to the mixture, and refluxed for 6 h. At the end of the reaction, it was cooled to RT, and a precipitate obtained, filtered, dried, washed with ethyl acetate, then recrystallized from EtOH.
Orange powder, yield 55%, m.p 258-260ᵒC, IR(KBr), (υ, cm-1):3070 cm-1 Ar(CH) str, 2920 and 2890 cm-1 asym & sym aliphatic (CH) str, 1608 cm-1 (C=N) str,1589 and 1489 cm-1 (C=C) str,1516 and 1344 cm-1 asym & sym (NO2) str, 858 cm-1 (out of plain CH benz.) bend; 1HNMR (300 MHz, DMSO-d6, δ= ppm): 10.12 (s,1H,NH); 8.84 (s,1H,CH=N); 8.45-6.53 (m, 8H,8Ar-H).
 |
Scheme 1: Synthesis of titled compounds (1-15) |
Antibacterial Activity
The new title compounds 4-15 were tested for their preliminary antibacterial activity, and measured using well diffusion technique26 in vitro, against three types of tested microorganisms: Gram-positive (G+ve) bacteria (S. aureus, Micrococcus luteus, and Bacillus pumilus), and two Gram-negative (G-ve) bacteria (Pseud. aeruginosa, and E.Coli), were clinically activated and maintained on nutrient agar medium for testing antibacterial activity, and potato dextrose agar medium for antifungal activity.27 Cefotaxime was used as a standard drug for antibacterial activity, while miconazole was used as a standard drug for antifungal activity, using a minimum inhibitory concentration, (MIC) of 1000 and 100μg/ml of the synthesized derivative in DMSO.
Results and Discussion
Chemistry
The hydrazide (1) obtained by refluxing of the starting material ethyl-4-aminobenzoate with hydrazine hydrate in absolute ethanol.
(1) characterized by FTIR, due to the formation of two doublet peaks for the primary amine of hydrazide, at 3271 and 3234 cm-1, also a prominent peak for the(C=O) amide stretching, at 1626 cm-1.
Two parent nuclei (2 and 3), had been synthesized from (1). Compound (2) obtained from stirring a methanolic solution of (1) with cyanogen bromide (CNBr), and sodium hydrogen carbonate, (NaHCO3) at ambient temperature, while (3) obtained from refluxing an ethanolic suspension of (1) with CS2 in the presence of KOH, the cyclization involved the formation of potassium salt of hydrazide, by acidification with 10% HCl. Each parent nucleus (2) and (3), displayed two peaks, at 3327 and 3224 cm-1 and 3315 and 3220 cm-1, respectively, that confirmed the presence of (NH2) group. In addition, (3) recorded a peak at 2557 cm-1 due to thiol group (SH), and a peak at 1290 and 1271 cm-1 for (2) and (3), respectively, due to asym (C-O-C) stretching, also other peaks at 1068 and 1065 cm-1, respectively, owing to sym (C-O-C) stretching, (oxadiazole ring formation). The 1HNMR spectrum for the parent nucleus (2) exhibited a singlet peak at δ=7.40 ppm, attributed to the primary aromatic-NH2 group, as well as, a peak appeared at δ=5.60 ppm, due to (NH2)-oxadiazole.
The four aromatic protons of (2), displayed at δ=6.85-6.50 ppm, as a multiplet.
The second parent nucleus (3), showed a peak at δ =7.78 ppm corresponds to the presence of primary NH2-group, while the four aromatic protons, for each (2) and (3), appeared at their expected region. See (exp. Part).
The cyclic imide derivatives (4-6) and (10-12), were synthesized in good yields, (85-95%), by thermal fusion of (2) or (3), with one of the three acid anhydrides, phthalic, succinic and maleic.
The IR spectrum of (4), displayed a distinct band at 3307 cm-1 owing to (OH) stretching of the carboxylic group, and indicates the opening of one imide ring, at one side of a ring fusion, besides that, a peak displayed at 1749 cm-1, due to (C=O) stretching of the carboxylic acid. Other peaks assigned for (C=O) amide stretching, at 1681 and 1651 cm-1, belong to the second fused imide ring. The 1HNMR spectrum for (4), exhibited a prominent peak, at δ =10.54 ppm, attributed to (OH) signal of the carboxylic acid, and confirms the opening of one of phthalic imide ring, the aromatic rings integrating for twelve protons, appeared at their expected aromatic region.
(5) showed a prominent IR band at 1664cm-1, due to conjugated (C=O) amide stretching, and 1HNMR displayed two signals, each as a doublet, appeared at δ=7.12 and 6.36 ppm, respectively, owing to the two protons of each maleimide ring.
The IR spectrum for (6) showed a characteristic carboxylic acid absorption, due to (OH) stretching at 3315 cm-1, and a peak displayed at 1725cm-1, assigned for (C=O) acid stretching. Other peaks displayed at 1701 and 1666 cm-1 belong to (C=O) stretching of amide, while 1HNMR analysis for (6) is similar to (4), exhibited a peak at δ= 12.10, as a singlet, due to (OH) signal of the carboxylic acid, also confirms the opening of one of succinimide ring, besides that, a peak integrating for two protons, belonging to (CH2) group, displayed at δ =2.76 ppm, as a triplet. The second aliphatic (CH2) group and the two (CH2) groups of succinimide ring were masked with DMSO–d6 peak.
(10) And (11) represent the product fusion, of each of phthalic anhydride, and maleic anhydride with (3), respectively, The NH-stretching of thioamide, for each, (10) and (11), displayed at 3225 and 3168 cm-1 respectively, also IR spectra recorded two peaks assigned for the (C=O) amide stretching, for (10) at 1739 and 1712 cm-1, while (11) displayed a (C=O) amide stretching , at 1687 cm-1 due to (conjugation).The1HNMR of (10) showed peaks, as multiplet, at the range of δ =8.06-7.69 ppm, due to eight aromatic protons, While (11) showed a peak at δ =10.68 ppm, due to NH-thioamide, also another two distinct signals, each attributed for one proton, appeared as a doublet, at δ=6.51 and δ=6.33ppm, respectively, (maleimide ring).
The IR spectrum for (12) is similar to (6), in which succinimide ring is opened during thermal fusion. A recorded band at 3450 cm-1, assigned for (OH) stretching of a carboxylic acid,(ring opening), a new peak at 1697 cm-1, due to (C=O) of the carboxylic acid stretching, While The 1HNMR showed a signal for (OH) of the carboxylic acid appeared at δ=14.60 ppm, in addition, two other signals, one displayed at δ=12.10 and another at 10.28 ppm, attributed to NH-thioamide and NH-amide.
Schiffʼs bases (7-9) and (13-15) synthesized by a conventional method involved, refluxing of an ethanolic or methanolic solution of (2) or (3), with aromatic aldehydes in an acidic medium (using glacial acetic acid).
The characteristic imine group displayed in IR spectra at 1606, 1576 cm-1 for (7), at 1653 and 1597cm-1 for (8), and at 1676 and 1597 cm-1 for (9), respectively. Also, (7) showed characteristic (OH) stretching at 3566 cm-1 and (Ar-O-CH3) absorption band, at 1252 cm-1, While for (8), a distinct peak due to (Ar-O-CH3), recorded at 1254 cm-1. Compound (9) exhibited a characteristic band, due to asym (NO2) stretching, at 1516 cm-1 and sym (NO2) stretching at 1344 cm-1, on the other hand, Schiff bases, are well characterized by 1HNMR spectroscopy, due to the appearance of imine group (CH=N), at δ= 8.50, 8.59 and8.42 ppm, for (7,8 and 9), respectively.
All the aromatic protons for (7-9) and (13-15), are well characterized and displayed at their expected aromatic regions. For more details, for the rest of the functional groups, see (exp. part).
Antimicrobial Evaluation
It’s evident from the data displayed in tables 1 and 2, compound (11) showed slight antibacterial activity against (Staph. aureus and Bacillus pumilus).
Compound (13) showed moderate activity towards tested Gram positive bacteria.
Among all synthesized compounds, (15) showed potent antibacterial activity against ( Micrococcus luteus), moderate antibacterial activity against ( Bacillus pumilus) , moderate antibacterial activity against (E. coli ) ,moderate antifungal activity against ( Saccharomyces cerevisiae) and slight antifungal activity against (Candida albicans) .
Table 1: The antibacterial activity of tested compounds (4-15).
|
Compd. No. |
Conc. μg/ml |
Staph. aureus |
Micrococcus luteus |
Bacillus pumilus |
Pseud. aeruginosa |
E.coli |
| Inhibition zone (mm) | ||||||
|
4 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
5 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
6 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
7 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
8 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
9 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
10 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
11 |
103 |
8.3 |
_ |
8.1 |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
12 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
13 |
103 |
10.6 |
10.5 |
11.5 |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
14 |
103 |
_ |
_ |
_ |
_ |
_ |
|
102 |
_ |
_ |
_ |
_ |
_ |
|
|
15 |
103 |
_ |
22.7 |
11.6 |
_ |
14.1 |
|
102 |
_ |
18 |
_ |
_ |
_ |
|
|
Cefot. |
103 |
38.0 |
68.0 |
33.0 |
35.6 |
39.0 |
|
102 |
31.8 |
63 |
25.8 |
30.8 |
32.12 |
|
|
DMSO |
_ |
_ |
_ |
_ |
_ |
_ |
Table 2: The antifungal activity of tested compounds (4-15).
|
Compd No. |
Conc. μg/ml
|
Saccharomyces cerevisiae |
Candida albicans |
||
|
|
Inhibition zone (mm)
|
||||
|
4
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
5
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
6
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
7
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
8
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
9
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
10
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
11
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
12
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
13
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
14
|
103 |
_ |
_ |
||
|
102 |
_ |
_ |
|||
|
15
|
103 |
14.2 |
8 |
||
|
102 |
_ |
_ |
|||
|
Miconazole |
103 |
15.8 |
32 |
||
|
102 |
14 |
30.8 | |||
|
DMSO (control) |
– |
– |
– |
||
(-)= No activity, slightly active (ZI =5-10 mm), moderately active (ZI= 10-15 mm), highly active (ZI= more than 15 mm),28,29 ZI-= Zone of inhibition.
Conclusion
A new oxadiazoles derivatives (imides and Schiff bases), derived from ethyl -4-amino benzoate, were successfully synthesized, by thermal fusion, and conventional methods, respectively, they were characterized and evaluated for their antimicrobial activities.
Compound (11) showed a slight activity against some of the Gram- positive bacteria, while (13) showed moderate activity against all Gram- positive bacteria used in this evaluation. Furthermore, (15) displayed a moderate to potent antibacterial activity, against different (Gram- positive and Gram-negative) bacteria, and also showed a slight to moderate antifungal activity.
Acknowledgment
We’re grateful to the College of Pharmacy-Dept. of Pharmaceutical Chemistry-University of Baghdad, for conducting the research, and many thanks to Dr. Raied M. Al-Sayab/College of Ibn -Al-Haitham, for Pure Science, for supporting 1HNMR analysis.
References
- Patel, Navin. B., Patel, Jaymin C., Synthesis and antimicrobial activity of 3- (1,3,4-oxadiazol-2-yl) quinazolin- 4(3H)-ones, Sci .Pharm. , 2010, 78,171–193 .
CrossRef - Panda, J.; Patro, V. J.; Panda, C. S.; Mishra J,and Mishra, J., Synthesis, characterization, antibacterial and analgesic evaluation of some 1,3,4- oxadiazole derivatives, Der Pharma Chemica, 2011,3(2), 485- 490.
- Nagalakshmi, G., Synthesis, antimicrobial and antiinflammatory activity of- 2,5-disubstituted-1,3,4-oxadiazoles. Indian J Pharm Sci., 2008, 70 (1), 49-55.
CrossRef - Rajak, H.; Kharya, M. D.; and Mishra, P., Synthesis of some novel oxadiazole and oxadiazoline analogues for their ant-iinflammatory activity. Yakugaku zasshi, 2007, 127 (10), 1757-1764.
CrossRef - Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li Q, Hu D; Xu, R., Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg Med Chem Lett., 2006, 16 (19), 5036-5040.
CrossRef - Tuma, MC.; Malikzay, A.; Ouyang, X.; Surguladze, D.; Fleming, J.; Mitelman, S.; Camara, M.; Finnerty, B.; Doody, J.; Chekler, EL.; Kussie, P.; Tonra, J., Antitumor activity of IMC-038525, a novel oral tubulin polymerization inhibitor. Transl Oncol, 2010, 3 (5), 318-325.
CrossRef - Banday, M. R.; Mattoo, R. H.; Rauf, A., Synthesis, characterization and anti-bacterial activity of 5-(alkenyl)-2-amino- and 2-(alkenyl)-5-phenyl-1,3,4-oxadiazoles. J. Chem. Sci., Indian Academy of Sciences 2010, 122 (2), 177–182.
CrossRef - Maslat, A. O.; Abussaud, M.; Tashtoush, H.; AL-Talib, M.; Synthesis, antibacterial, antifungal and genotoxic activity of Bis-1,3,4-oxadiazole derivatives, Pol J Pharmacol., 2002, 54, 55-59.
- Shah, H. P.; Shah, B. R.; Bhatt, J. J.; Desai, N. C.; Trivedi, P. B.; Undavia, N. K., Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles as potential antimicrobial, anticancer and anti-HIV agents, Indian J. Chem., 1998, 37 (B), 180-182.
- Maheshwari, R.; Chawla, P.; Saraf, S., Comparison between antioxidant activity of 2,5-disubstituted 1,3,4-oxadiazoles containing heteroaromatic ring and aromatic ring at 2nd position, Med Chem Res.,2010, (3), 1-6.
- Musmade, D. S.; Pattan, S. R.; and Manjunath, S. Yalgatti., Oxadiazole a nucleus with versatile biological behavior, Int. J. Pharm. Chem.,2015 ,5,11-20.
- Kadi, AA.; El-Brollosy, NR.; Al-Deeb, OA.; Habib, EE.; Ibrahim, TM.; El-Emam, AA., Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur J Med Chem., 2007,42(2),235–242. [PubMed].
CrossRef - Mickevičius, V.; Vaickelioniene, R.; Sapijanskaite, B., Synthesis of substituted 1,3,4-oxadiazole derivatives., Chem. Heterocycl. Compd., 2009,45(2),215–218.
CrossRef - Bentiss, F.; Lagrenee, M.; A new synthesis of symmetrical 2,5-disubstituted 1,3,4-oxadiazoles. J. Heterocycl. Chem., 1999,36(4),1029–1032.
CrossRef - Liras, S; Allen, MP.; Segelstein, BE., A mild method for the preparation of 1,3,4-oxadiazoles: triflic anhydride promoted cyclization of diacylhydrazines. Synth. Commun.,2000,30(3),437–443
CrossRef - Gomes, D.; Borges, CP.; Pinto, JC., Study of the synthesis of poly(4,4′-diphenylether-1,3,4-oxadiazole) in solutions of poly(phosphoric acid) Polymer. 2001,42(3),851–865.
CrossRef - Frański, R., Biological activities of the compounds bearing 1,3,4-oxa(thia)diazole ring. Asian J. Chem., 2005, 17(4),2063–2075
- Parekh, J.; Chanda, S.; In vitro antimicrobial activity of Trapa natans L. fruit rind extracted in different solvents. Afr. J. Biotechnol., 2007,6(6),766–770.
- Yao, X.; Jericho, M.; Pink, D.; Beveridge; T., Thickness and elasticity of gram-negative murein sacculi measured by atomic force microscopy.J. Bacteriol., 1999, 181(22),6865–6875.[PMC free article] [PubMed]
- Jian, Zhang.; Muthaiah, S.; Subhash, C.G.;and Soon, H.H., Synthesis of cyclic imides from simple diols, Angew. Chem. Int. Ed., 2010, 49, 6391 –6395 .
CrossRef - Nadjet, Rezki.; Amjad, M. Al-Yahyawi.; Sanaa, K. Bardaweel .; Fawzia, F. Al-Blewi.; and Mohamed, R. Aouad ., Synthesis of novel 2,5-disubstituted-1,3,4-thiadiazoles clubbed 1,2,4-triazole, 1,3,4-thiadiazole, 1,3,4-oxadiazole and/or Schiff Base as potential antimicrobial and anti-proliferative agents, Molecules ,2015, 20, 16048-16067.
CrossRef - E.Ghanem.; S. Al-Hariri.; M. Bin Alia., Synthesis and characterization of novel nematic liquid crystal, Damascus University j. for basic and appl. Sci., 2010,2(26),193-213.
- Henryk, Foks.; Mieczyslaw, Janowiec.; Zofia, Zwolska.; and Ewa, Augustynowicz-Kopeć ., Synthesis and tuberculostatic activity of some (4-phenylpiperazin-1- ylmethyl)-1,3,4-oxadiazole and (4-phenylpiperazin-1-ylmethyl)-1,2,4-triazole derivatives. Acta Pol Pharm., 2004, 61(6)473-476 .
- S. Magaldi; S. Mata-Essayag.; C. Hartung de Capriles; C. Perez; M.T. Colella.; Carolina, Olaizola.; Yudith, Ontiveros., Well diffusion for antifungal susceptibility testing, Int. J. of Infectious Diseases. 2004, 8,39-45.
CrossRef - Valgas, C.; Souza, SM.; Smânia, EF.; Smânia, Jr A., Screening methods to determine antibacterial activity of natural products. Braz. J Microbiol., 2007, 38(2),369-380.
CrossRef - Dabholkar, VV.; and Gavande, RP., Synthesis and antimicrobial activities of novel 1, 4-benzothiazine derivatives. Arabian J. Chem., 2016, 9,225-S229.
CrossRef - Ali, PS.; Meshram, JS.; and Raut, RD., Theoretical and synthetic approach towards the biology of some novel monobactam induced sulphonamides: assessing biology through coupling of active ingredients. Jordan J. Chem., 2011, 6(1),153-164.

This work is licensed under a Creative Commons Attribution 4.0 International License.









