Synergistic Effect Between Gum Exudates of Eucalyptus Globles and 2,6-Diphenyl-3-Methylpiperidin-4-one on Corrosion Inhibition of MS in 1N HCl
Dheenadhayalan S1 , Roja R2
, Roja R2 , Nijarubini V3
, Nijarubini V3 and Mallika J1
and Mallika J1
1,2Department of Chemistry, PSG College of Arts and Science, Coimbatore 641014, India.
3Department of Chemistry, Dr. N.G.P Arts and Science College, Coimbatore 641048, India.
Corresponding Author E-mail: jmchempsg2016@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340545
Article Received on : 05-05-2018
Article Accepted on : 19-08-2018
Article Published : 11 Oct 2018
Gum exudates of eucalyptus globles (GEG) was identified as green inhibitor for MS dissolution in 1N HCl using gravimetric method at 303-323K. Efficiency of GEG was synergistically increased with addition of 2,6-diphenyl-3methyl-piperidin-4-one (3MDPP). The binary combination of GEG and 3MDPP shows maximum inhibition potency and their Sθ value is >1 indicating that synergism exists between GEG and 3MDPP. Mechanism of inhibition of inhibitors on MS is physisorption and it obeys Langmuir’s isotherm. Polarization and impedance measurements confirm that inhibitors act as mixed type.
KEYWORDS:Binary Mixture; GEG; Mild Steel; 3MDPP; Synergism
Download this article as:| Copy the following to cite this article: Dheenadhayalan S, Roja R, Nijarubini V, Mallika J. Synergistic Effect Between Gum Exudates of Eucalyptus Globles and 2,6-Diphenyl-3-Methylpiperidin-4-one on Corrosion Inhibition of MS in 1N HCl. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Dheenadhayalan S, Roja R, Nijarubini V, Mallika J. Synergistic Effect Between Gum Exudates of Eucalyptus Globles and 2,6-Diphenyl-3-Methylpiperidin-4-one on Corrosion Inhibition of MS in 1N HCl. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50595 |
Introduction
In many industries corrosion is a major problem for materials during acid pickling or chemical cleaning. Recently plant gum exudates are found to be non-toxic green corrosion inhibitors for various metals in different media due to its good adhesive and polymeric nature.1-13 These exudates protect the metal surfaces by adsorption through physical or chemical bonding. But inhibition performance and stability of these gum exudates are found to be less significant at higher temperature. Some researchers use metal cations and halide ions as external additive to enhance their inhibition performance.14,15 A thorough survey of literature reveals that, no work has so far been done on the influence of organic compounds as synergist for corrosion inhibition behavior of plant gum exudates and also their stability. So the present work aims to study the corrosion inhibition properties of gum exudates of eucalyptus globles (GEG) towards the corrosion of MS in 1N HCl at 303-323K for 1 hour immersion period. At higher temperature the stability and efficiency of GEG was enhanced by addition of external synergist (3MDPP). In acidic medium, 3MDPP act as very good corrosion inhibitor.16 Therefore the influence of 3MDPP on the inhibition character of GEG towards MS is studied in 1N HCl at 303-323K. Because binary combination of GEG and 3MDPP system exhibits excellent inhibition potency than individual components and also this mixture completely control or reduce the rate of dissolution of MS even at very higher temperature.
Methodology
The gum exudates of eucalyptus globles (GEG) was collected in local area of Nilgiri in Tamil nadu, India. The collected gum is purified following the reported procedure.17 The GEG solution was prepared with distilled water. The 3MDPP was synthesized using the reported procedures.18,19 Laboratory grade HCl was used as an aggressive medium. The composition of C, Mn, P and S in the MS (%wt) are 0.2, 1.0, 0.25 and 0.025 respectively. The MS coupons of dimensions of 25 × 10 × 1 mm were used for the present study, following ASTM practice standard G-31 procedure.20
The corrosion parameters are derived using equation (1-3).
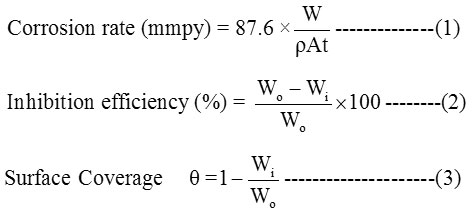
Where, W, ρ , A , t , Wo and Wi represents mass loss (g), density of MS (gcm˗3), area of the coupon (cm2), immersion time (h),weight loss of MS without and with inhibitor respectively.
A conventional three electrode assembly is taken for polarization and impedance studies. The experiment is performed with 100 ml of test solution at 30°C. A SCE, platinum and MS were used as reference, counter and working electrodes respectively. The scan rate and potential range of 1 mVs-1 and +200 to −200 mV were employed. Extrapolation method was used to measure the corrosion current and potential. Inhibition potency was determined using equation 4.
Inhibition potency = (i°corr – icorr)/ i°corr X 100 ———- (4)
Where, i°corr and icorr are the corrosion current density in the absence and presence of inhibitor.
Impedance data were derived in the frequency range of 10-1x 10-5 KHz at OCP employing 10 mV amplitudes of AC signal. Zview software was used to obtain Rct and Cdl values. The values of Rct are used to calculate inhibition potency by employing equation 5.
Inhibition potency = (Rct – R◦ct) /Rct X 100 ———- (5)
Where, Rct and R◦ct are the charge transfer resistance with and without inhibitor.
Result and Discussion
Effect of GEG
The inhibition parameters studied (303-323K) for GEG towards MS in 1N HCl medium clearly reveal that GEG controls the MS dissolution (Table 1). The inhibiting potency of GEG on MS in 1N HCl directly varies with its concentration and it is mainly due to presence of carbohydrate, protein and polyphenols. These molecules are electrochemically active and interact with the MS surface through their labile electrons resulting in the development of film on MS surface. At higher temperature the inhibition efficiency of GEG decreases. This may be due to rapid movement of HCl molecules towards the MS surface and thus detaches the GEG molecules from the surface.
Effect of 3MDPP
The corrosion inhibiting ability of 3MDPP at room temperature directly varies with its concentration (Table 1). The carbonyl group and ring nitrogen in 3MDPP are probable groups that are able to form protective film on the MS. The simultaneous involvement of carbonyl group and ring nitrogen cannot be taken into account because it would be possible only through the attainment of boat conformation which is highly strained and 3MDPP exist in chair conformation.21 Among the two anchoring site, the ring nitrogen is the most probable one due to its less electronegativity. At higher temperature, desorption of 3MDPP from the MS surface occurs causing a decrease in inhibition potency.
Synergistic Influence of 3MDPP on GEG
The binary combination of GEG and 3MDPP acts as efficient inhibitor for MS in 1N HCl. The increasing potency of binary mixture may be due to synergism existing between GEG and 3MDPP. The value of Sθ is evaluated using the relationship given by Aramaki.22 The values of Sθ are >1 for all studied combinations indicating the pronounced inhibition potency resulting from the injection of 3MDPP to GEG in testing solution (Table 1). The inhibition nature of the binary mixture is higher than the individual components and stable up to 323K. This can be explained as follows: The non bonding electrons present on the ring nitrogen atom of 3MDPP act as nucleophile. In acidic medium the positively charged metal surface attracts the non bonding electrons of 3MDPP towards itself. The adsorbed 3MDPP molecules on the metal surface act as intermediate bridge between MS surface and GEG. The adsorbed 3MDPP molecule holds the GEG through the several hydrogen bonds formed between carbonyl group of 3MDPP and phenolic/ hydroxyl/ carboxyl groups of GEG and hence prevent the outward movement/ desorption of 3MDPP and the metal dissolution.
Table 1: Corrosion parameters for GEG, 3MDPP and binary mixture on MS in 1N HCl.
| T (K) | C (ppm) | I E (%) | CR (mmpy) | Sθ | |
| GEG | 3MDPP | ||||
| 303 | Blank | 0.0820 | – | ||
| 10 | – | 14.29 | 0.0703 | – | |
| 50 | – | 25.81 | 0.0609 | – | |
| 100 | – | 32.26 | 0.0556 | – | |
| 150 | – | 36.41 | 0.0522 | – | |
| 200 | – | 42.40 | 0.0473 | – | |
| – | 50 | 41.94 | 0.0476 | – | |
| – | 250 | 56.22 | 0.0359 | – | |
| – | 500 | 75.58 | 0.0200 | – | |
| – | 750 | 81.11 | 0.0155 | – | |
| 50 | 50 | 64.06 | 0.0295 | 1.06 | |
| 50 | 250 | 79.26 | 0.0170 | 1.04 | |
| 50 | 500 | 84.79 | 0.0125 | 1.20 | |
| 50 | 750 | 90.78 | 0.0076 | 1.18 | |
| 313 | Blank | 0.1652 | – | ||
| 10 | – | 12.36 | 0.1448 | – | |
| 50 | – | 21.74 | 0.1293 | – | |
| 100 | – | 29.52 | 0.1164 | – | |
| 150 | – | 34.10 | 0.1089 | – | |
| 200 | – | 39.13 | 0.1006 | – | |
| – | 50 | 36.38 | 0.1051 | – | |
| – | 250 | 53.32 | 0.0771 | – | |
| – | 500 | 66.82 | 0.0548 | – | |
| – | 750 | 79.18 | 0.0344 | – | |
| 50 | 50 | 59.27 | 0.0673 | 0.98 | |
| 50 | 250 | 80.09 | 0.0329 | 0.94 | |
| 50 | 500 | 83.98 | 0.0265 | 1.06 | |
| 50 | 750 | 87.87 | 0.0200 | 1.15 | |
| 323 | Blank | 0.2446 | – | ||
| 10 | – | 10.97 | 0.1644 | – | |
| 50 | – | 19.17 | 0.1263 | – | |
| 100 | – | 25.50 | 0.0866 | – | |
| 150 | – | 26.12 | 0.0541 | – | |
| 200 | – | 30.14 | 0.2178 | – | |
| – | 50 | 32.77 | 0.1977 | – | |
| – | 250 | 48.38 | 0.1822 | – | |
| – | 500 | 64.61 | 0.1807 | – | |
| – | 750 | 77.90 | 0.1709 | – | |
| 50 | 50 | 50.70 | 0.1206 | 1.02 | |
| 50 | 250 | 69.55 | 0.0745 | 0.97 | |
| 50 | 500 | 81.14 | 0.0461 | 1.03 | |
| 50 | 750 | 82.84 | 0.0420 | 1.17 | |
Thermodynamic and Kinetics Studies
The mechanism of adsorption process of any molecule can be predicted with the help of Arrhenius (6) and Transition state (7) equations.
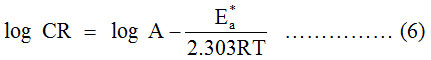
Where, CR, A, Ea*, R and T represents corrosion rate, frequency factor, activation energy, universal gas constant and absolute temperature respectively. The plot of (log CR) vs (1000/T) (Figure 1) forms a straight line with the slope (-Ea*/2.303 R) from which the values Ea* can be calculated (Table 2). The values of ΔH* and ΔS* are calculated using equation (7).
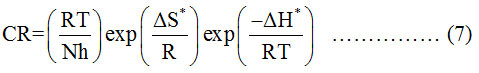
Where, N, h, ΔS* and ΔH* represents the Avogadro’s number, Planck’s constant, entropy of activation and enthalpy of activation respectively. A straight line with slope (-ΔH*/2.303R) and intercept [log(R/Nh) + (ΔS*/2.303R)] is obtained by plotting log (CR/T) vs (1000/T) (Figure 2). The values of ΔH* and ΔS* are calculated and included in the Table (2). The values of Ea* and ΔH* for GEG, 3MDPP and binary mixture are much higher than blank (Table 2) which indicates physisorption. The positive sign of ΔH* reflect the endothermic nature of MS dissolution. The negative sign of ΔS* for both systems confirms the decrease in disorderness during the conversion of reactant to activated complex which is the rate determining step.23
Table 2: Activation parameters for GEG, 3MDPP and binary mixture on MS in 1N HCl.
| Inhibitor | C (ppm) | Ea* (kJ mol-1) | ΔH* (kJ mol-1) | -ΔS* (J mol-1) |
| GEG | Blank | 44.57 | 41.97 | 127.00 |
| 10 | 46.12 | 43.52 | 123.17 | |
| 50 | 48.07 | 45.47 | 117.91 | |
| 100 | 48.43 | 45.83 | 117.52 | |
| 150 | 50.64 | 48.03 | 110.86 | |
| 200 | 52.38 | 49.78 | 105.92 | |
| 3MDPP | 50 | 50.55 | 47.95 | 111.74 |
| 250 | 51.26 | 48.66 | 111.85 | |
| 500 | 59.77 | 57.17 | 88.26 | |
| 750 | 50.97 | 48.37 | 119.70 | |
| Binary mixture | 50 + 50 | 57.40 | 54.80 | 93.27 |
| 50 + 250 | 59.99 | 57.39 | 89.82 | |
| 50 + 500 | 53.27 | 50.67 | 114.10 | |
| 50 + 750 | 69.82 | 67.21 | 63.63 |
 |
Figure 1: Arrhenius plots for GEG, 3MDPP and binary mixture on MS in 1N HCl. |
 |
Figure 2: Transition plots for GEG, 3MDPP and binary mixture on MS in 1N HCl. |
Adsorption Isotherm
Adsorption of an inhibitor onto the metal surface in acidic medium depends on structure and conformation of inhibiting molecule, electrochemical potential on the metal and temperature of the medium. The mechanism of adsorption can be predicted by using Henry, Frendlich, Langmuir, Frumkin, and Temkin isotherm etc. Langmuir’s isotherm is found to be the best description for present study and it is given by

Where, Kads is the equilibrium constant. The (ΔG°ads) is calculated following the equation.
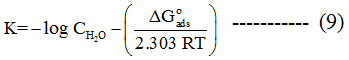
The Langmuir’s plot (Figure 3) for tested inhibitor system gave a straight line which indicates the monolayer adsorption. From the intercept of the plot the values of Kads were obtained which is used to calculate the values of ΔG°ads (Table 3). In general, ΔG°ads values ≤ -20 kJmol-1 are suggestive of physisorption, while beyond this value (-40 kJmol-1) is said to be chemisorptions.23 The calculated values of ΔG°ads for GEG, 3MDPP and binary mixture are in the range of 16.63-17.33, 15.66-15.77 and 18.37-19.22 kJmol-1 respectively (Table 3). These results confirm the spontaneity of the reaction and the inhibitor molecule are adsorbed through physisorption process.
Table 3: Adsorption parameters for GEG, 3MDPP and binary mixture on MS in 1N HCl.
| Inhibitor | T(K) | Intercept | K (mol-1) | -ΔG◦ads (kJ mol-1) |
| GEG | 303 | 0.0755 | 13.2425 | 16.63 |
| 313 | 0.0942 | 10.6143 | 16.60 | |
| 323 | 0.0876 | 11.4094 | 17.33 | |
| 3MDPP | 303 | 0.1061 | 9.4221 | 15.77 |
| 313 | 0.1322 | 7.5637 | 15.72 | |
| 323 | 0.1627 | 6.1451 | 15.66 | |
| Binary mixture | 303 | 0.0379 | 26.3775 | 18.37 |
| 313 | 0.0345 | 28.9818 | 19.22 | |
| 323 | 0.0543 | 18.4049 | 18.61 |
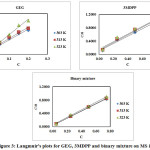 |
Figure 3: Langmuir’s plots for GEG, 3MDPP and binary mixture on MS in 1N HCl. Click here to View figure |
Tafel’s Polarization Studies
The corrosion inhibition behavior of GEG, 3MDPP and binary mixture towards the MS corrosion in 1N HCl were studied using Tafel’s method and the parameters icorr, Ecorr, ba and bc were derived. Analysis of Table 4 clearly shows that icorr value decreases with increasing the concentration of GEG whereas 3MDPP shows minimum icorr value. The binary mixture gives very least icorr value. Generally, solution inhibited by cathodic or anodic compounds show higher Ecorr value and mixed type inhibitor show lower Ecorr value when compare with uninhibited solution.24,25 Ecorr values for the studied inhibitor system is low and it can be viewed as mixed type, predominantly controlled by anodic reaction.
Table 4: Polarization parameters for GEG, 3MDPP and binary mixture on MS in 1N HCl
| Inhibitor | -Ecorr (mV/SCE) | icorr (mA/cm2) | ba (mV/dec) | bc (mV/dec) | IE (%) |
| Blank | -0.477 | 0.001612 | 0.1472 | 0.0785 | |
| 10 ppm GEG | -0.479 | 0.001312 | 0.1403 | 0.0773 | 18.61 |
| 50 ppm GEG | -0.476 | 0.00047 | 0.1178 | 0.0622 | 70.84 |
| 100 ppm GEG | -0.473 | 0.000419 | 0.1176 | 0.0633 | 74.00 |
| 150 ppm GEG | -0.475 | 0.000239 | 0.1044 | 0.0576 | 85.17 |
| 200 ppm GEG | -0.491 | 0.000186 | 0.1101 | 0.0815 | 88.46 |
| 500 ppm 3MDPP | -0.360 | 6.72E-05 | 0.1540 | 0.1136 | 95.83 |
| Binary mixture | -0.490 | 5.09E-05 | 0.1137 | 0.0847 | 96.84 |
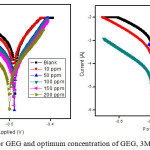 |
Figure 4: Tafel’s plots for GEG and optimum concentration of GEG, 3MDPP and binary mixture. |
Impedance Studies
It is one of the most suitable method to predict the formation of film/layer onto the metal surface. The impedance studies were performed to derive Rct and Cdl values for MS in 1N HCl in the presence of GEG, 3MDPP and binary mixture (Table 5). Their corresponding Nyquist plots are shown in Figure (5). Analysis of data clearly reveals that the value of Rct increases and Cdl value decreases in the presence of tested inhibitor systems. The influence is more pronounced in the case of binary inhibitor system. This may be due to the formation of week hydrogen bonding between 3MDPP and GEG molecule. Due to large molecular size of the GEG pushes the already adsorbed 3MDPP close to the MS surface which decreases the Cdl values and increases inhibition efficiency.26
Table 5: Impedance parameters for GEG, 3MDPP and binary mixture on MS in 1N HCl.
| Inhibitor | Rct (Ω cm2) | Cdl (μF cm-2) | IE (%) |
| Blank | 07.05 | 61.3 | |
| 10 ppm GEG | 11.36 | 86.6 | 37.94 |
| 50 ppm GEG | 31.30 | 77.7 | 77.47 |
| 100 ppm GEG | 37.80 | 82.1 | 81.34 |
| 150 ppm GEG | 51.62 | 78.1 | 86.34 |
| 200 ppm GEG | 92.66 | 69.6 | 92.39 |
| 500 ppm 3MDPP | 268.99 | 20.7 | 97.37 |
| Binary mixture | 322.47 | 51.6 | 97.81 |
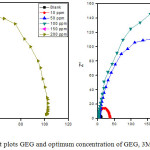 |
Figure 5: Nyquist plots GEG and optimum concentration of GEG, 3MDPP and binary mixture. Click here to View figure |
Conclusion
GEG and 3MDPP are very good corrosion inhibitors for dissolution MS in 1N HCl and their inhibiting ability increases with increasing its concentration but decreases with rise in temperature.
Binary combination of GEG and 3MDPP shows excellent inhibiting potency and also prevent the metal dissolution at higher temperature.
The value Sθ for binary mixture is >1 confirms the synergism existing between GEG and 3MDPP.
The calculated values of Ea*, ΔH* and ΔG◦ads for GEG, 3MDPP and binary mixture conclude a physisorption mechanism and it obeys Langmuir’s isotherm.
Tafel’s polarization study reveals that tested inhibitors act as mixed type.
Increasing Rct value and decreasing Cdl value in the presence of GEG, 3MDPP and binary mixture confirms the formation of film on MS.
Conflict of Interests
There is no conflict of interests.
Reference
- Paul Ocheje Ameh. Inter. J. Met. 2015, 2015, 1-13.
- Paul Ocheje Ameh. Inter. J. Mod. Che. 2012, 2(1), 28-40.
- Nnabuk Okon Eddy.; Udo J. Ibok.; Paul O. Ameh.; Nsor O. Alobi.; Musa M. Sambo. Chem. Eng. Comm. 2014, 201, 1360-1383.
- Anjali Peter.; Sanjay K. Sharma.; Ime Bassey Obot. J. Anal. Sci. Tech. 2016, 7(26), 1-15.
- Paul Ocheje Ameh.; Nnabuk Okon Eddy. Res. Chem. Inter. Med. 2014, 40, 2641-2649.
CrossRef - Paul Ocheje Ameh.; Ladan Magaji.; Takuma Salihu. Afric. J. Pu. App. Chem. 2012, 6(7), 100-106.
- Umoren, S.A.; Ekanem, U.F. Chem. Eng. Comm. 2010, 197, 1339-1356.
CrossRef - Umoren, S.A.; Obot, I.B.; Ebensob, E.E.; Obi-Egbedi. N.O. Desal. 2009, 247, 561-572.
CrossRef - David E. Arthur.; Adebiyi Adedayo.; Gerald Igelige.; Edwin Ogwuche. Amer. Chem. Sci. J. 2014, 4(6), 847-854.
- Nnabuk O. Eddy.; Anduang O. Odiongenyi.; Paul O. Ameh.; Eno E. Ebenso. Int. J. Electrochem. Sci. 2012, 7, 7425 – 7439.
- Hamza Bentrah .; Youssoul Rahali.; Abdelouahad Chala. Corr. Sci. 2014, 82, 426-431.
CrossRef - Mohammad Mobin.; Marziya Rizvi. Carbo. Poly. 2016, 136, 384-393.
CrossRef - Mobin, M.; Alam Khan, M. J. Dis. Sci. Tech. 2013, 34, 1496-1506.
CrossRef - Umoren, S.A.; Ebenso, E.E. Pig. Res. Tech. 2008, 37(3), 173-182.
CrossRef - Brindha, T.; Mallika, J. Orient. J. Chem. 2015, 31(2), 741-752.
CrossRef - Glory Tharial Xavier.; Brindha Thirumalairaj.; Mallika Jaganathan. Inter. J. Corros. 2015, 2015, 1-15.
- Malarvizhi Manickam.; Dheenadhayalan Sivakumar.; Brindha Thirumalairaj.; Mallika Jaganathan. Adva. Phy. Chem. 2016, 2016, 1-12.
- Balasubramanian, M.B.; Padma, N. Tetrahed. 1963, 19(12), 2135-2143.
CrossRef - Noller, C.R.; Baliah, V. J. Ameri. Chem. Soci. 1948, 70(11), 3853-3855.
CrossRef - ASTM practice standard G-31, “Standard practice for laboratory immersion corrosion testing of metals”, ASTM International, 2004.
- Selvaraj, K.; Manjula, N.; Mallika, J. Trans. Met. Chem. 2001, 26, 224.
CrossRef
- Aramaki, K.; Haghwara, M.; Nishihar, H.; Corro. Sci. 1987, 27, 487.
CrossRef
- Shukla, S.K.; Ebenso, E.E. Inter. J. Electrochem. Sci. 2011, 6, 3277.
- Umoren, S.A. Port. Electrochim. Acta. 2009, 27(5), 565-577.
CrossRef
- Li, W.H.; He, Q.; Zhang, S.T.; Pei, C.L.; Hou, B. J. App. Electrochem. 2008, 38, 289.
CrossRef - Goudarzi, N.; Peikari, M.; Zahiri, M. R.; Mousavi, H. R. Arch. Metal. Mat. 2012, 57, 845.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









