Potentiometric Studies of Binary Complexes of Transition Metal Ions with Lauric Acid
Govindarajan Kavitha , Parasuraman Vijaya Rohini and Sunkubangaru Sudarsan Alwar
, Parasuraman Vijaya Rohini and Sunkubangaru Sudarsan Alwar
Department of Chemistry, D. G. Vaishnav College, University of Madras.
Corresponding Author E-mail: kavitha.rajsekhar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340564
Article Received on : 05-04-2018
Article Accepted on : 15-08-2018
Article Published : 02 Oct 2018
The potentials of binary complexes of the Lauric acid ligand with various biologically vital divalent metal ions such as Mn(II), Co(II), Ni(II), Cu(II)and Zn(II) have been studied in 50% DMSO-water mixture at constant ionic strength by Bjerrum method. Hydrogen ion concentrations were measured and the formation of binary complexes was inferred from the corresponding potentiometric pH-titration curves. The metal to ligand mole ratio was maintained at 1:5 to facilitate the maximum number of ligands to the metal ion. The dissociation constant has been determined by the half-integral method and pointwise method and were found in good agreement with each other. The Metal-Lauric acid stability constants have been calculated and compared with the results.
KEYWORDS:DMSO -Water Mixture; Ph-Metric Method; Stability Constant
Download this article as:| Copy the following to cite this article: Kavitha G, Rohini P. V, Alwar S. S. Potentiometric Studies of Binary Complexes of Transition Metal Ions with Lauric Acid. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Kavitha G, Rohini P. V, Alwar S. S. Potentiometric Studies of Binary Complexes of Transition Metal Ions with Lauric Acid. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50127 |
Introduction
Studies on binary complexes of vital biological ligands in aqueous and aqueous-organic media have received reasonable attention in recent years. The nonaqueous and aqueous-organic solvents are used instead of water due to their wide range of applications. The current work is significant for understanding the role of the aqueous-organic solvent medium for complex formation. This part of the research work gives an insight about ion-solvent interaction and the interaction among the solvents.
Dimethyl sulfoxide(DMSO) is well known polar aprotic solvent. Aqueous-DMSO, binary solvent medium is used as a cryoprotective agent. In the aqueous-DMSO mixture, the water structure is enhanced, the water from the coordination sphere of metal ions is removed, and they are made more approachable towards the ligands resulting in the increased stability of the complexes. But the competing behaviour of DMSO as a coordinating solvent may reduce the stability of the complex. Hence the increased and decreased stability of the complexes may be attributed to the structure forming and competing for the behaviour of DMSO.1
Lauric acid, dodecanoic acid, is a saturated fatty acid present in coconut milk and coconut oil.2 It is widely used in the synthesis of soaps and cosmetics. Using the freezing-point depression method, the molecular weight of chemical compounds could be determined. Lauric acid is metabolized in the human body, into monolaurin which has significant antifungal and antimicrobial properties.3 It destroys the micro-organisms by disrupting their lipid membranes.
The essential metals such as manganese, Cobalt, Nickel, Copper and Zinc play an important role in biological processes. Cobalt is at the active center of coenzymes called cobalamin. Nickel is present in the enzyme, urease, which is found in the wide range of plant species. Copper is found to have antibacterial properties.
Hence, potentiometric studies of Lauric acid with selective transition metal ions have been carried out. In the present study, the dissociation constant, stability constants of lauric acid with the metal ions have been reported in aqueous- DMSO medium. The binary complexes are more suitable for the transport of metals in bio fuels due to their increased stability and the availability of metals would be enhanced in biological systems because of their lower stability.
Comprehensive research work has been carried out to have a quantitative approach in the field of complexation studies. A lot of literature survey4-9 has been carried out in this field still it is observed that little information is available about the proton-ligand constant10 of lauric acid in methanol-water and ethanol-water medium and the stability constants of the ligand with metal ions are not available.
Materials and Methods
All chemicals used were of the analar grade. The metal salt solution, ligand solutions were prepared using deionized water and standardized by standard volumetric methods. The pH of the titrating mixture has been measured using a combined glass electrode attached to a Systronics microprocessor-based pH meter (QJ-666B) with accuracy ± 0.01. The calibration of pH meter has been carried out with suitable buffers. 50% v/v DMSO-water medium was used. A measured quantity of potassium nitrate solution was added to maintain 0.1M ionic strength. The concentrations of lauric acid and metal ions solutions used were 20 x 10-4 mol dm-3, and 4 x 10-4 mol dm-3. The three solution mixtures of volume 25 mL were prepared by taking the following solutions:(a) Free HNO3 (b) HNO3 + ligand (c) HNO3 + ligand + metal ion solution respectively.
Above three solution mixtures were titrated against standardized sodium hydroxide (0.098 M). The pH reading was observed after the addition of aliquots of alkali in all the cases. The recorded pH was plotted against the volume of sodium hydroxide. The acid curve, ligand curve and metal complex titration curve (Fig.1) were obtained. In the present work, Calvin-Bjerrum method, as modified by Rossotti-Rossotti11was used, as the method is simple and reliable.
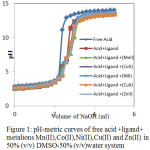 |
Figure 1: pH-metric curves of free acid + ligand + metalions Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) in 50% (v/v) DMSO-50% (v/v) water system. |
Results
From the acid and ligand curves, the formation number, nA (average number of protons associated with the ligand) was calculated at different pH values. Using the Irving-Rossotti expression, the proton-ligand formation number, nA was estimated (Table 1).
Table 1: Pointwise method for calculation of pK in 50%(v/v) DMSO-50%(v/v)water system.
|
pH |
V1 |
V2 |
∆V=V2-V1 |
nA |
|
5.6 |
2.249 |
2.388 |
0.319 |
0.7246 |
|
5.8 |
2.251 |
2.410 |
0.159 |
0.6849 |
|
6.0 |
2.262 |
2.442 |
0.180 |
0.6435 |
|
6.2 |
2.266 |
2.482 |
0.216 |
0.5722 |
|
6.4 |
2.270 |
2.524 |
0.254 |
0.4971 |
|
6.6 |
2.276 |
2.556 |
0.280 |
0.4456 |
|
6.8 |
2.282 |
2.582 |
0.300 |
0.4062 |
|
7.0 |
2.289 |
2.602 |
0.325 |
0.3569 |
|
7.2 |
2.294 |
2.619 |
0.327 |
0.3530 |
Proton-ligand dissociation constant of Lauric acid at 270C in 50%(v/v) DMSO-50%(v/v)water system
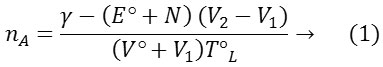
Where γ denotes number of replaceable protons, E°, N, T°L are the concentration of acid and sodium hydroxide(0.098 M) and the ligand respectively. V° is the initial volume of the reaction mixture. The data of nA obtained at various pH values along the horizontal difference (V2-V1) for representative systems. The dissociation equilibrium of the ligand can be shown as.
![]()
The pK values of ligand from the formation numbers were determined using pointwise calculations. It is also estimated by the half-integral method, by noting the corresponding pH for nA = 0.5 from proton-ligand formation curves nA versus pH (Fig.2). The accurate value of pK which is determined by pointwise calculations is found to be almost the same as the value calculated by the half-integral method (Table 2).
Table 2: pK values of Lauric acid.
|
Ratio of DMSO –water mixture |
pK(Half integral method) |
pK(pointwise calculation method) |
|
50%(v/v) DMSO-50%(v/v)water system |
6.38 |
6.44 |
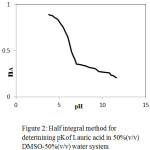 |
Figure 2: Half integral method for determining pKof Lauric acid in 50%(v/v) DMSO-50%(v/v) water system. |
The increase in pK values in the binary solvent medium than pure water shows that DMSO plays a significant role to increase the basicity. The solvation effect and the bulk solvent structure might have been influenced by the dissociation of the ligand. From the metal complex titration curves. The average number of metal ions associated with the ligand (n) for different pH values have been calculated. The stability constants of metal complexes were obtained by the half-integral method. The n values have been calculated by using the expression.
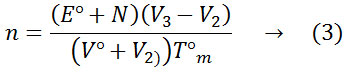
Where N°, E°, V° and V2 have the similar importance as that of equation (1).V3 is the volume of alkali added and Tm is the concentration of the metal ion.
The metal-ligand stability constants of various complexes have been determined using n values (fig 3) and were tabulated. (Table 3).
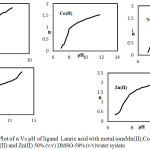 |
Figure 3: Plot of n Vs pH of ligand Lauric acid with metal ionsMn(II),Co(II), Ni(II),Cu(II) and Zn(II) 50% (v/v) DMSO-50% (v/v)water system. Click here to View figure |
Table 3: Metal-ligand stability constants of Lauric acid with transition metal ions in 50%(v/v) DMSO-50%(v/v)water system.
|
System |
log K1 |
log K2 |
logK1-log K2 |
logK1/logK2 |
|
Mn (II) – L |
3.097 |
2.610 |
0.487 |
1.187 |
|
Co (II) – L |
3.077 |
2.520 |
0.557 |
1.221 |
|
Ni (II) – L |
3.087 |
2.620 |
0.467 |
1.178 |
|
Cu (II) – L |
2.997 |
2.600 |
0.397 |
1.153 |
|
Zn (II) – L |
3.067 |
2.500 |
0.567 |
1.227 |
Discussion
The present work explains the complexation of selective divalent transition metal ions with lauric acid in the aqueous-DMSO medium. The dissociation and stability constants have been determined to understand the behavior of the ligand and its interaction with metal ions in the aqueous-DMSO medium. The ligand was a weak complexing agent. The change in color (without precipitation) concerning pH value of solution and deviation of ligand curve from metal ion curve also shows the commencement of complex formation.
The difference between log K1 and log K2 values signifies the formation of complexes. Since the difference between log K1 and logK2 is small, both 1:1 and 1:2 complexes are formed simultaneously and but not in a stepwise manner. On adding DMSO to water as co-solvent, there is a decrease in the dielectric constant of the solvent medium and the interaction among solvent molecules are found to be increased.12
The less value of dielectric constant is the reason for the lowering in solvation of the metal ions in DMSO, resulting in higher attraction force which in turn, approaches the ligand to viable coordination of metal ion resulting in enhanced stability of the complex in DMSO.13 Hence the electrostatic forces of attraction between two oppositely charged species were significant, and therefore the stability of the complexes are more in the aqueous-organic solvent mixtures than in purely aqueous medium. This could be attributed to the hydrogen bonding ability of water and the solvating tendency of DMSO.14 The higher value of the ratio (log K1/logK2) for Co(II) and Zn(II)-ligand complexes denotes the stability of the above complexes is more compared to Mn(II), Ni(II)and Cu(II)-ligand complexes.
These studies help to study the pharmaceutical applications of lauric acid in the future.
Acknowledgments
The authors wish to thank the Head and faculty of the Department of Chemistry, D.G.Vaishnav for their support in carrying out this work.
References
- Belay,H.H.;Sailaja, B.B.V.; Rao, G.N.Der Pharma Chemica,2015,7(5),147-156
- Beare-Rogers,J.;Dieffenbacher,A.;Holm,J.V.Pure and Applied Chemistry,2001,73(4),685-744
CrossRef - Nakatsuji,T.;Kao,M.C.;Fang ,J.Y.;Zoubilis,C.C.;Zhang,L.;Gallo,R.L.;Huang,C.M.The Journal of Investigative Dermatology, 2009,129(10),2480-8
CrossRef - HarminderKaur;AnitaSingla. International Journal of Pure &Applied Chemistry,2011,6,149-153.
- Tayade,D.T.;Wadekar,A.B.;International Journal of Applied Research,2015,01,640-642
- Hussain.Chemistry Journal,2012,02,206-209
- Abdalazeem,A.O.;Elmugdad.A.International Journal Of Advanced Chemistry,2015,03, 6-13
- AvinashRamteke;MarutilNarwade. Archives of Applied Science Research,2013,5,231-237.
- Vijayarohini,P.; Kavitha,G.; Bangaru Sudarsan Alwar,S.DerPharmaChemica,2017,9(22):25-28
- Rahman,M.A.;.Ghosh,A.K..;Bose,N.A.;Journal of Chemical Technology and Biotechnology,1979,29,158-162
CrossRef - Irving, H.M.;Rossetti, H. S.Journal of Chemical Society,1954,2904.
CrossRef - Srinivas,N.;Manikyamba,P.Rasayan Journal of chemistry,2008,1,315-321
- Thanavelan,R.;Ramalingam,G.;Manikandan,G.;Thanikachalam,V.Journal of Saudi Chemical Society,2014,18,227-233
CrossRef - Vijaykumar,S.I.;Ashwini,K.S.Polyhedron,2003,22,569-574.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









