Microwave Assisted-Hydrothermal Synthesis of Nickel Ferrite Nanoparticles
Mohamed H. H. Mahmoud1,2 and Mahmoud M. Hessien1,2
and Mahmoud M. Hessien1,2
1 Department of Chemistry, College of Science, Taif University, Taif, Saudi Arabia.
2 Central Metallurgical Research and Development Institute (CMRDI), P.O. Box: 87 Helwan, Cairo, Egypt.
Corresponding Author E-mail: mheshamm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340546
Article Received on : 25-08-2018
Article Accepted on : 30-09-2018
Article Published : 16 Oct 2018
Nanomagnetic ferrite materials are of great technological importance in several industries due to their high performance, ease of preparation and low cost. The ferrite properties are based on composition, structure and methods of preparation. Nickel ferrite, NiFe2O4, was prepared by the simple microwave assisted-hydrothermal method. Nickel chloride and ferric chloride solutions (stoichiometric ratio of 1: 2 respectively) were mixed, the pH was raised to 10.5 and the mixture was heated at 180 °C in a closed Teflon vessel using a microwave oven at different periods of time (2 - 24 h). The formed powders were examined by XRD, TEM, and VSM. The intensity of nickel-ferrite in the XRD patterns increased with time owing to increase in crystallinity of the formed phase. The TEM images showed that, the size was in the range of 20-40 nm and contents of fine particles noticeably decreased with increasing reaction time to 4-6 hrs and contents of more regular cubic particles are formed. The NiFe2O4 magnetization was continuesly increased with raising the heating time from 2h (9 emu/g) to 24 h (43 emu/g) which may be due to the high purity and crystallinity of the formed NiFe2O4. The results showed that the properties of the formed ferrite can be tailored by controlling the heating time. Microwave assisted co-precipitation followed by hydrothermal digestion resulted in a substance of good homogeneity and crystallinity at a short time.
KEYWORDS:Hydrothermal; Magnetic Properties; Nanoparticles; Nickel Ferrite
Download this article as:| Copy the following to cite this article: Mahmoud M.H.H, Hessien M.M. Microwave Assisted-Hydrothermal Synthesis of Nickel Ferrite Nanoparticles. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Mahmoud M.H.H, Hessien M.M. Microwave Assisted-Hydrothermal Synthesis of Nickel Ferrite Nanoparticles. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50869 |
Introduction
Synthesis of nano and functional materials and their doping with rare and noble metals have attracted attention of many researchers.1,2 Magnetic nano-materials are characterized by their enormous physical properties, ease of preparation and low cost of raw materials. They have important technological applications in modern industries such as RF generators, medical treatment of cancer and others. Spinel ferrites ( MFe2O4, M can be Co, Mn, Cu, Ni, Zn or their mixture), have improved properties suitable for advanced applications due to their exclusive stability and structure.3,4 These materials are used in diverse applications, such as gas sensors, catalysis, magnetic media and pigments.5,6 Nano-crystalline spinel ferrite particles receive considerable attention due to their novel magnetic behavior7-9
Nickel ferrite (NiFe2O4) has been studied widely and reported as a magnetic matter.10-15 It was prepared using the typical ceramic method by calcination of finely ground oxides at high temperature (>1200°C ) followed by pressing and sintering.16 Major disadvantages of this method are coarser particle size, inhomogeneity, excess of impurities generated from milling and low magnetization.17 High-temperature self-propagation,18,19 combustion synthesis [20], and high temperature decomposition of polyethylene glycol or citrate21-23 have been also used for synthesis of ferrites.
Recently, numerous nonconventional soft synthetic routes based on wet-chemical reactions have been used to prepare nano-crystalline ferrites such as glass crystallization24 co-precipitation,25 hydrothermal and hydrothermal mechanical alloying,26-29 microemulsion30 and sol–gel methods31,18 C. Surig, K.A. Hempel and D. Bonnenborg, J. IEEE Trans. Magn. 30 (1994), p. 4092.
The hydrothermal process (aqueous reaction under pressure) is a low temperatures method for synthesizing powders in nano-size. Synthesis by the hydrothermal technique has attracted attention because it is an efficient route to produce high crystallized, with a narrow size distribution. The main advantages of this process were in controlling morphology, particle size, and other characteristics by controlling several reaction parameters.32 By this method, a single phase of nano-ferrites powder at low temperature, narrow size distribution and uniform morphology can be produced. Nejati and Zabihi studied the effect of surfactant and heating temperature on the formation of NiFe2O4 particles.33 However, several other parameters can control the properties of formed nickel ferrite.
Method of heating by normal or microwave oven and the heating duration may have pronounce effect on the prepared ferrite. Microwave radiation can improve the chemical reactions compared with thermal heating. Microwave energy is absorbed by the reaction mixtures, in the presence of polar compound, and heat the inner part of the mixture. The heat transports from the inner into the boundaries (wall of the vessel). This heat transfers again from the boundaries into the interior, and this cycle continuously repeated forming the so called conductive-convective heat transfer. These behaviors usually accelerate the reactions and improve the product.34 In the present work nickel ferrite, was synthesized using the simple hydrothermal method by heating the mixture of hydrated nickel chloride and ferric chloride solutions in a microwave oven at different periods of time. The produced powders were characterized by TEM, XRD, and VSM.
Experimental
Stoichiometric amounts of nickel chloride and ferric chloride were dissolved in 200 cm3 of distilled water and the pH was raised to 10.5 by adding drops of saturated NaOH solution and the mixture was homogenized. This pH value was selected to ensure that all nickel and iron are co-precipitated in their hydrated form. The formed precipitate mixture was homogenized, poured into a Teflon vessel, sealed and placed in a microwave device (CEM Mars Microwave Reaction / Extraction System) for heating. The applied digestion conditions were as follows: power 800, ramp time 10 min, temperature 180 ○C, duration time 2, 4, 6, 10 and 24 h. After elapse of the duration time, the mixture cooled gradually to room temperature and the formed material filtered, washed three times with distilled water and then dried at 70 ○C for 24 hours. The dried powder were crushed gently with a spatula and kept in plastic bags for characterization by XRD, TEM, and VSM.
Crystalline phases were detected by X-ray diffraction, XRD, using a Brucker axis D8 diffractometer in the range 2θ from 10 to 80o. VSM (Vibrating Sample Magnetometer 9600-1 LDJ, USA) was utilized to measure the magnetic features of the prepared materials at a maximum applied field of 7 kOe. Saturation magnetization (Ms), coercivity (Hc) and remanence magnetization (Mr), were measured. TEM (Transmission Electron Microscope, JTEM-1230, Japan, JEOL) was used to detect and measure the shape and particle size of the prepared materials.
Results and Discussion
X-ray Diffraction, XRD
The prepared materials have been examined by the XRD instrument to identify their phase composition, confirm the formation of the NiFe2O4 phase, its progress with increasing the retention time and existence of any impurities. Figure. 1 shows the patterns of X-ray diffraction of the prepared materials. Patterns of the standard NiFe2O4 ( JCPDS No. 10-325 ) was also plotted in Figure 1[33]. It can be noticed from this figure that a known cubic structure of NiFe2O4 phase was detected in all samples. The major beaks were well defined in all samples with neglected trace of Fe2O3 (hematite). The diffraction peaks of NiFe2O4 were weak at short retention time and the intensity increased at longer times. This behavior is correlated with increasing the crystallinity with time. The main diffraction peak ( (311) appears at 2 Theta of 35.7○) is shown in Table 1. It can be noticed that the intensity of the main beak was low at short time of 2 h (16.5) and increased at longer time till 10 h (32) and then slightly decreased. Thus, heating the mixture for a period from 4 h to 10 h is sufficiently suitable for preparation of the targeted nickel ferrite phase with high crystallinity. It can be also concluded here that, the technique of microwave-assisted hydrothermal digestion of co-precipitation salts resulted in a substance of good homogeneity and crystallinity at a short time such as 4 h.
 |
Figure 1: XRD spectra of the synthesized nickel ferrite at different conditions. NiFe2O4, Fe2O3 |
Table 1: Relation between retention time and intensity of the main peak of NiFe2O4 at 180 oC
|
Retention Time, h |
Intensity of the main peak, counts |
|
2 |
16.5 |
|
4 |
23.5 |
|
6 |
25 |
|
10 |
32 |
|
24 |
25 |
Transmission Electron Microscope, TEM
The nickel ferrite powders prepared at different periods of time (from 2 hours to 24 hrs) have investigated by TEM and their images are shown in Figures 2-6. It can be noticed by investigating these TEM micrographs that at a short time of two hours (Fig. 2), there are two distinguished kinds of nano-particles, a cubic-like and a finer elongated. This may indicate that two hours is an insufficient period of reaction time, where the starting fine particles reactants of nickel and iron oxides may still exist, and/or nuclei of the target nickel ferrite is in the beginning of formation stage. Contents of fine particles noticeably decreased with increasing reaction time to 4 hrs and contents of more regular cubic practices are formed (Fig. 3). The fine particles almost disappeared at longer reaction time of 6 hours, and contents of the more uniform cubic nanoparticles were found (Fig.4). By increasing the reaction time to 10 and 24 hours, clear uniform cubic particles were only noticed (Fig. 5 and 6). These TEM images showed an average grain-size of approximately 20-40 nm, at 4 – 24 hrs.
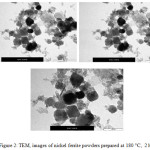 |
Figure 2: TEM, images of nickel ferrite powders prepared at 180 ○C, 2 h Click here to View figure |
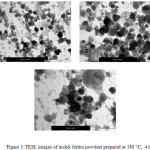 |
Figure 3: TEM, images of nickel ferrite powders prepared at 180 ○C, 4 h |
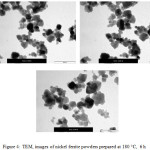 |
Figure 4: TEM, images of nickel ferrite powders prepared at 180 ○C, 6 h |
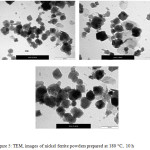 |
Figure 5: TEM, images of nickel ferrite powders prepared at 180 ○C, 10 h Click here to View figure |
 |
Figure 6: TEM, images of nickel ferrite powders prepared at 180 ○C, 24 h Click here to View figure |
Magnetic Behavior
The magnetic properties of the nano-crystalline NiFe2O4 powder was measured using VSM, and the hysteresis loops were plotted in Figure 7. This figure shows plots of magnetization (M) as a function of the applied field (H) at different times of synthesis (2 – 24 h) of NiFe2O4 at 180 ○C. Magnetic behavior initiates from occupation of Fe3+ at octahedral and tetrahedral sites and Ni2+ in octahedral sites can explain the inverse spinel and magnetic characteristics of the NiFe2O4 .35,36 Hysteresis loops in Figure 7 are characteristic for soft magnetic matter. The “S” shape curves indicated the presence of superparamagnetic particles.35
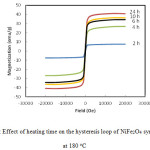 |
Figure 7 : Effect of heating time on the hysteresis loop of NiFe2O4 synthesized at 180 oC Click here to View figure |
It is clear from Figure 7 that the increase in synthesis time has a pronounced effect on the magnetic properties. The magnetization of the NiFe2O4 was continueously increased with time. It reached ≈9 emu/g after 2h and raised to 43 emu/g after 24 h of heating time. Such a pronounced magnetization of the prepared ferrite after 24 h may be due to the noticeable phase purity and high crystallinity of NiFe2O4, as confirmed in SEM and XRD results shown above.
Conclusions and Remarks
Nickel ferrite NiFe2O4 particles in nano-size scale were synthesized using microwave assisted-hydrothermal method at 180 oC for different periods of time (from 2 to 24 h). The prepared samples had an average particle size in the range of from 20 to 40 nm, as observed by investigation using TEM and XRD. The nickel ferrite powders were all considered as superparamagnetic materials. The values of coercivity and saturation magnetization were increased with increasing the period of synthesis time. The results also showed that the phase of nickel-ferrite in the formed nanoparticles was increased with synthesis time as the magnetic properties increased.
Acknowledgement
The Authors would like to express their gratitude to the deanship of scientific research, Taif University, KSA, for financially supporting this work under research project no. 1-436-4558.
Conflict of interest statement
The authors whose names are listed immediately below certify that there is no any conflict of interest regarding this work.
Mohamed H. H. Mahmoud,
Mahmoud M. Hessien
References
- Mahmoud, M. H. H.; Ismail, Adel A; Sanad, M. M. S. Chem. Eng. J. 2012, 187, 96-103.
CrossRef - Mostafa, N. Y.; Mahmoud, M. H. H.; Heiba, Z. K. Hydromet. 2013, 139, 88-94.
CrossRef - Son, S.; Taheri, M.; Carpenter, E.; Harris, V. G.; McHenry, M. E. J. Appl. Phys. 2002, 91, 7589-7591.
CrossRef - Cedeño-Mattei, Y.; Perales-Pérez, O. Microelectron. J. 2009, 40, 673-676.
- Maensiri, S.; Masingboon, C.; Boonchom, B.; Seraphin, S. Scripta materialia, 2007, 56, 797-800.
CrossRef - Sugimoto, M. J. Am. Ceram. Soc. 1999, 82, 269-280.
CrossRef - Liu, C.; Zou, B.; Rondinone, A. J.; Zhang, Z. J. J. Am. Chem. Soc. 2000, 122, 6263-6267.
CrossRef - Hessien, M. M. Journal of J. Magn. Magn. Mter. 2008, 320, 2800-2807.
CrossRef - Hessien, M. M.; Rashad, M. M.; El-Barawy, K. J. Magn. Magn. Mter. 2008, 320, 336-343
CrossRef - Albuquerque, A. S. D. ; Ardisson, J. D. ; Macedo, W. A. D. A. ; Lopez, J. L.; Paniago, R. ; Persiano, A. I. C. J. Magn. Magn. Mter. 2001, 226, 1379-1381.
CrossRef - Mozaffari, M.; Amighian, J. J. Magn. Magn. Mter. 2003, 260, 244-249.
CrossRef - Korotcenkov, G. Sens. Actuators B. 2005, 107, 209-232.
CrossRef - Costa, A. C. F. M. ; Silva, V. J. ; Cornejo, D. R. ; Morelli, M. R. ; Kiminami, R. H. G. A.; Gama, L. J. Magn. Magn. Mter. 2008, 320, e370-e372.
CrossRef - Azizi, A.; Sadrnezhaad, S. K. Ceram. Inter. 2010, 36, 2241-2245.
CrossRef - Nawale, A. B.; Kanhe, N. S.; Patil, K. R.; Bhoraskar, S. V.; Mathe, V. L.; Das, A. K. J. Alloy. Compd. 2011, 509, 4404-4413.
CrossRef - Satyanarayana, L.; Reddy, K. M.; Manorama, S. V. Sens. Actuators B 2003, 89, 62-67.
CrossRef - Satyanarayana, L.; Reddy, K. M.; Manorama, S. V. Mater. Chem. Phys. 2003, 82, 21-26.
CrossRef - Baba, P. D.; Argentina, G.; Courtney, W.; Dionne, G.; Temme, D. IEEE Trans. Mag. 1972, 8, 83-94.
CrossRef - Kim, C. S.; Kim, W. C.; An, S. Y.; Lee, S. W. J. Magn. Magn. Mter. 2000, 215, 213-216.
CrossRef - Hu, P. ; Yang, H. B.; Pan, D. A.; Wang, H.; Tian, J. J. ; Zhang, S. G. ; Volinsky, A. A. J. Magn. Magn. Mter. 2010, 322, 173-177.
CrossRef - Peng, C. H.; Hwang, C. C.; Hong, C. K.; Chen, S. Y. Mater. Sci. Eng., B, 2004, 107, 295-300.
CrossRef - Hwang, C. C.; Tsai, J. S.; Huang, T. H.; Peng, C. H.; Chen, S. Y. J. Solid State Chem. 2005, 178, 382-389.
CrossRef - Mouallem-Bahout, M.; Bertrand, S.; Pena, O. J. Solid State Chem. 2005, 178, 1080-1086.
CrossRef - Sato, H., Umeda, T. J. Mater. Trans. 1993, 34, 76-81.
CrossRef - Radwan, M.; Rashad, M. M.; Hessien, M. M. J. Mater. Process. Tech. 2007, 181, 106-109.
CrossRef - Ding, J.; Miao, W. F.; McCormick, P. G.; Street, R. J. Alloy. Compd. 1998, 281, 32-36.
CrossRef - Bućko, M. M.; Haberko, K. J. Eur. Ceram. Soc. 2007, 27, 723-727.
CrossRef - Huo, J.; Wei, M. Mater. Lett. 2009, 63, 1183-1184.
CrossRef - Wang, L. ; Ren, J.; Wang, Y. ; Liu, X.; Wang, Y. J. Alloy. Compd. 2010, 490, 656-660.
CrossRef - Liu, C.; Zou, B.; Rondinone, A. J.; Zhang, Z. J. J. Phys. Chem. B 2000, 104, 1141-1145.
CrossRef - Kim, C. S.; Yi, Y. S.; Park, K. T., Namgung, H., Lee, J. G. J. Appl. Phys. 1999, 85, 5223-5225.
CrossRef - Wang J.; Huang M.; Zhong Q.; Lin J.; Wu J. J. of Funct. Mat. 2009, 40, 1442−1444.
- Nejati, K.; Zabihi, R. Chem. Cent. J. 2012, 6, 23.
CrossRef - Nuechter, M., Mueller, U., Ondruschka, B., Tied, A., Lautenschlaeger, W. Chem. Eng. Technol., 2003, 26, 1207-1216.
CrossRef - Kodama, R. H.; Berkowitz, A. E.; McNiff Jr, E. J.; Foner, S. Phys. Rev. Lett. 1996, 77, 394-397.
CrossRef - Manova, E.; Tsoncheva, T.; Estournes, C.; Paneva, D.; Tenchev, K.; Mitov, I.; Petrov, L. Appl. Catal. A 2006, 300, 170-180.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









