Kinetics of Lycopene Degradation in Sunflower and Grape Seed Oils
Antonio Zuorro1, Roberto Lavecchia1 , Erenio González2 and Viatcheslav Kafarov3
, Erenio González2 and Viatcheslav Kafarov3
1Department of Chemical Engineering, Materials and Environment, Sapienza University, Roma, Italy.
2Center of Process Analysis, Faculty of Chemistry and Pharmacy, Central University of Las Villas, Villa Clara, Cuba.
3Department of Chemical Engineering, Universidad Industrial de Santander, Bucaramanga, Colombia.
Corresponding Author E-mail: roberto.lavecchia@uniroma1.it
DOI : http://dx.doi.org/10.13005/ojc/340502
Article Received on : 07-08-2018
Article Accepted on : 03-09-2018
Article Published : 12 Oct 2018
The stability of lycopene in two vegetable oils, sunflower seed oil (SSO) and grape seed oil (GSO), was investigated by analysing the carotenoid degradation kinetics in the temperature range of 10–40°C. A tomato oleoresin containing 6% (w/w) of lycopene was used to prepare lycopene-enriched oil samples. Analysis of kinetic data showed that lycopene degradation follows first-order kinetics, with an apparent activation energy of 70.7 kJ mol–1 in SSO and 69 kJ mol–1 in GSO. The estimated half-life of lycopene was found to depend on oil type and storage temperature. At 20°C, it varied between 59 and 122 days, while at 4°C it was comprised between 302 and 650 days. At all temperatures, lycopene was more stable in SSO than in GSO, which is likely due to the higher content of antioxidant compounds in SSO.
KEYWORDS:Degradation Kinetics; Lycopene; Functional Foods; Vegetable Oils
Download this article as:| Copy the following to cite this article: Zuorro A, Lavecchia R, González E, Kafarov V. Kinetics of Lycopene Degradation in Sunflower and Grape Seed Oils. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Zuorro A, Lavecchia R, González E, Kafarov V. Kinetics of Lycopene Degradation in Sunflower and Grape Seed Oils. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50687 |
Introduction
Lycopene (ψ,ψ–carotene), the carotenoid responsible for the deep red color of ripe tomatoes and tomato products, is one of the most potent natural antioxidants.1 Chemically, lycopene is an acyclic tetraterpenic hydrocarbon with 13 C–C double bonds, 11 of which are conjugated (Fig. 1). This high degree of conjugation gives this carotenoid strong antioxidant and free radical-quenching properties.
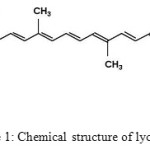 |
Figure 1: Chemical structure of lycopene |
In human plasma, lycopene is found at concentrations of 0.2 to 1 nmol mL–1 and accounts for at least 10% of all carotenoids.2 Humans are unable to synthesize carotenoids and, therefore, lycopene can only be acquired from dietary sources. Once absorbed, it accumulates in various organs, especially the liver, the lung, the colon and the prostate.3 Many studies have shown that an inverse relationship exists between plasma lycopene levels and the incidence of oxidative stress-related diseases, such as prostate, lung, and stomach cancer.4 Evidence has also been provided that the consumption of lycopene-rich tomato products can reduce cellular DNA damage in smokers and type-2 diabetics.5 These beneficial effects have been ascribed to the combined presence of lycopene and other tomato phytochemicals, such as β-carotene, phytoene and phytofluene, which are known to synergistically enhance the antioxidant activity of lycopene.
In view of the above observations, the possibility of producing new functional foods enriched with lycopene has been the object of increasing interest. Recent studies have demonstrated the feasibility of enriching vegetable oils with lycopene. For example, Benakmoum et al.6 showed that edible oils with a lycopene content of up to about 3 mg/100 g can be obtained using tomato peels or tomato puree as lycopene source. Zuorro and Lavecchia7 used the peels of ripe tomatoes to incorporate lycopene into rice bran and seed oils. In another study by the same research group, a tomato seed oil produced from the seed fraction of tomato processing waste was enriched with a lycopene extract obtained from the peel fraction of the same waste.8 Recently, a lycopene-rich avocado oil was produced by supercritical extraction from avocado pulp and tomato pomace.9
Although vegetable oils fortified with lycopene can be regarded as valuable functional foods with potential health benefits, there are some issues that need to be considered prior to their commercial introduction. One of the most important is related to the poor stability of lycopene in oils. In fact, while the native membrane-bound carotenoid is relatively stable, it becomes easily susceptible to oxidation once it is released into a nonpolar environment.10
The aim of the present study was twofold: (a) to investigate the possibility of using a standardized commercial tomato oleoresin containing lycopene and other tomato phytochemicals to produce a lycopene-enriched vegetable oil, and (b) to evaluate the stability of lycopene in the oil. Sunflower and grape seed oils were used as a base for preparing the functional oils and the kinetics of lycopene degradation in these products was studied in the temperature range of 10–40 °C.
Materials and methods
Tomato oleoresin and vegetable oils
Lyc-O-Mato®, an oleoresin produced from whole tomatoes, was obtained from LycoRed Natural Products Industries Ltd. (Beer-Sheva, Israel). The oleoresin contained about 6% (w/w) lycopene and minor amounts of phytoene, phytofluene, β-carotene and tocopherol.
Grape seed oil (GSO) was provided by ACEF (Fiorenzuola d’Arda, PC, Italy) while sunflower seed oil (SSO) was purchased from a local market. The oils were stored in the dark at room temperature until use.
Preparation of lycopene-enriched oil samples
Lycopene-enriched oil samples were obtained by inclusion of the tomato oleoresin in the oils. A mother solution of the carotenoid in each oil was first prepared by placing 40 mg of oleoresin and 50 g of oil into screw-top glass flasks. The flasks were magnetically stirred for about 45 min at low speed and room temperature (20 ± 2 °C). Then, the resulting suspension was centrifuged at 7,000 × g for 5 min and the oil was analysed for lycopene content.
Lycopene assay
Lycopene concentration in the oils was determined spectrophotometrically. Measurements were made in the wavelength range 400–600 nm with a double-beam UV/Vis spectrophotometer (Perkin-Elmer Lambda 25). Absorption spectra showed the three characteristic peaks of lycopene at around 456, 483 and 518 nm (Fig. 2). The peak at 518 nm was used to quantify the concentration of the carotenoid.
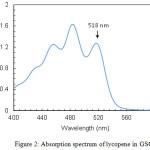 |
Figure 2: Absorption spectrum of lycopene in GSO |
Lycopene degradation studies
Lycopene degradation experiments were carried out in a forced-air incubator (FTC 90E, VELP Scientifica, MB, Italy) with the temperature controlled to ± 0.2 °C. Pure oil samples were poured into 50-mL glass flasks and allowed to equilibrate at the desired temperature (10, 25 or 40 °C). Then, an appropriate amount of the mother solution of lycopene was added to the pure oil to obtain the desired carotenoid concentration. The flasks were gently shaken by hand for homogenization and left unstirred for the duration of the experiment in order to reproduce real storage conditions. At selected times, aliquots were withdrawn and analysed for lycopene content. All experiments were repeated at least twice and the results were averaged.
Results and Discussion
The stability of lycopene in two vegetable oils, SSO and GSO, was investigated in order to provide preliminary information on the formulation of new functional products with the health benefits of lycopene.
As is known, the highly unsaturated hydrocarbon structure of lycopene makes it easily susceptible to degradation. Studies on the stability of lycopene, particularly under food processing and storage conditions, showed that the degradation process is very complex and proceeds through both isomerization and oxidation.11,12 Isomerization reactions convert all-trans lycopene to mono- and poly-cis isomers, while oxidation leads to the cleavage of the lycopene molecule with the formation of a variety of products, such as apo-lycopenals, apo-lycopenones, apo-carotendials and lower molecular weight compounds.13 According to the generally accepted mechanism of reaction, lycopene degradation can be schematized as a reversible isomerization step followed by an irreversible oxidation step.14 Under typical storage conditions, isomerization is the main reaction and does not affect the total lycopene content. However, the rate and extent of this reaction, as well as of oxidation, depend on several environmental factors, the most important being temperature, light and oxygen.
Moderate heating has limited effect on total lycopene, affecting mainly isomerization, while under more severe conditions an enhancement in oxidation is observed, with an increase in lycopene losses.11,15 Interestingly, in fats, the trans– and cis-lycopene forms are protected against oxidation and the isomerization reactions are slowed down.14
As observed in studies on lycopene-containing food products and model systems, irradiation of lycopene by light causes a decrease in total lycopene and produces more or less significant changes in the trans– to cis-isomer distribution.15,16
Finally, the presence of oxygen has a detrimental effect of lycopene, its loss increasing with the levels of dissolved oxygen.11,14 A study performed on lycopene dissolved in the oil phase of oil-in-water emulsions showed that the extent of lycopene degradation under oxygen saturation was three times higher than in the absence of oxygen.17 Accordingly, it is necessary to minimize contact with air in order to preserve lycopene activity.
The kinetics of lycopene degradation in SSO and GSO was investigated at 10, 25 and 40 °C by analysing the dependence of the absorbance at 518 nm (A518) on time. At every temperature and initial carotenoid concentration, a linear trend was observed for the function A518(t). Exemplary results, referring to lycopene in GSO at 40°C and two different initial concentrations, are shown in Fig. 3.
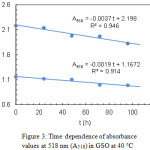 |
Figure 3: Time dependence of absorbance values at 518 nm (A518) in GSO at 40°C |
The mass balance equation for lycopene in a batch system at constant temperature and volume can be written as:
![]()
where c is the carotenoid concentration, t is the time and –rd is the degradation rate. The experimental c values were determined from the absorbance at the selected wavelength using a molar extinction coefficient7 of 1.303×105 M–1 cm–1. Then, the degradation rate at each temperature and initial lycopene concentration was evaluated from the slope of the regression line; this means to assume –dc/dt ≃ –Δc/Δt. Plotting the degradation rates against the average lycopene concentrations gave the results shown in Fig. 4. As can be seen, –rd varied linearly with c, which is indicative of a first-order kinetics:
![]()
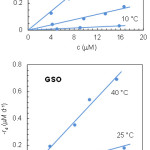 |
Figure 4: Kinetic plots of lycopene degradation in SSO and GSO. |
where kd is the apparent first-order rate constant. This parameter was determined from the slope of the regression lines, giving the estimates listed in Table 1. As apparent from the scatter plot in Fig. 5, the first-order model provided a good description of lycopene degradation, the average error on degradation rate being about 0.012 μM d–1. In addition, a statistical analysis of residuals, performed as described elsewhere,18 showed no deviations from linear regression assumptions.
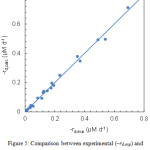 |
Figure 5: Comparison between experimental (–rd,exp) and calculated (–rd,calc) lycopene degradation rates. |
Examination of Table 1 reveals that, at every temperature, lycopene degradation in SSO was less pronounced than in GSO. As expected, an increase in temperature resulted in faster degradation.
To describe the temperature dependence of kd, the Arrhenius equation was used:
![]()
where A is the pre-exponential factor and Ea is the apparent activation energy. The estimated activation energies in SSO and GSO were, respectively, 70.73 and 68.96 kJ mol–1. From the temperature dependence of the apparent rate constants in the two oils, the lycopene half-life (τ1/2), i.e., the time required for the initial lycopene concentration to be reduced by 50%, was calculated as:
![]()
Fig. 6 shows the trend of the function τ1/2 (T) between 0 and 40°C, which represents the temperature range of major interest for the storage of food products. At each temperature, the half-life of lycopene was greater (i.e., the carotenoid was more stable) in SSO than in GSO. However, differences in stability decreased as the temperature increased. Above approximately 30°C, the two curves were very close to each other.
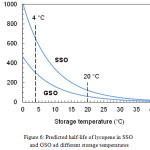 |
Figure 6: Predicted half-life of lycopene in SSO and GSO ad different storage temperatures. |
The different stability of lycopene in the two oils is a reflection of their different fatty acid composition and antioxidant content. In particular, it is known that the higher the degree of unsaturation of an oil, the faster its oxidative degradation.19 Lipid oxidation can lead to the formation of highly reactive species, such as alkyl and peroxyl radicals, which, in turn, can increase the susceptibility of lycopene to degradation.20
An examination of the fatty acid profiles of SSO and GSO, according to Codex Alimentarius,21 reveals that they are characterized by very similar amounts of saturated fatty acids (10–12%), with small differences in the distribution between monounsaturated and polyunsaturated fatty acids. As a result, it can be assumed that the different stability of lycopene in the two oils is mainly due to the type and amount of endogenous antioxidants. In vegetable oils, different classes of antioxidant compounds can be found, including phenolics, carotenoids, tocopherols and tocotrienols.22,23
Tocopherols and tocotrienols, commonly known as vitamin E, are the main antioxidants in seed oils.24 They have similar chemical structures, being derivatives of 6-chromanol and differing only in the number and position of methyl substituents in the phenolic ring.25 According to Codex Alimentarius21, their range of values is from 440 to 1520 mg kg–1 in SSO and from 240 to 410 mg kg–1 in GSO. Therefore, the greater stability of lycopene in SSO could be due to the higher amount of tocopherols. Studies performed on the degradation of lycopene26 and β-carotene27 in the presence of tocopherols showed that these antioxidants can protect carotenoids from oxidative damage. Regeneration of the biologically active carotenoid by tocopherols is thought to proceed by an electron transfer mechanism.28 Interestingly, tocopherols were found to enhance the chemopreventive activity of lycopene against breast cancer29 and its anti-inflammatory properties.30 This provides strong support to the development of lycopene-enriched seed oils as novel functional foods.
In Table 2, the half-life values for lycopene degradation at two significant storage temperatures, 4 and 20 °C, are reported. At the lower temperature, the half-life is about 650 days in SSO and 300 days in GSO. At 20°C, these values are reduced by about five times. In Table 2, a comparison is also made with the results obtained in a previous study7 using tomato peel extracts as lycopene source. It can be seen that the stability of lycopene in the two oils is greatly affected by the carotenoid source. In particular, in SSO the half-life of lycopene from tomato peel extracts is about 15% lower than that from tomato oleoresin. In GSO the situation is reversed, with an increase of about 50%. An explanation of these results can be found in the different characteristics of the two lycopene sources. In fact, while the oleoresin used in this work was produced from whole tomatoes, consisting mainly of the fruit pulp, the tomato extract was obtained from the fruit peels. Whole tomatoes and tomato peels differ considerably in composition and structure.31,32 Furthermore, in the oleoresin a lipid fraction derived from the seeds of the fruit is also present, with potential effects on the stability behavior of lycopene. In fact, tomato seed oil contains high levels of γ-tocopherol.8 This compound and its main degradation products, γ-tocopherol biphenyl-dimer and γ-tocopherol ether-dimer, are very effective as antioxidants and act by a mechanism different from that of α-tocopherol.33 Accordingly, it can be hypothesized that the combined presence of α- and γ-tocopherol, as in the case of SSO enriched with the oleoresin, can stabilize lycopene to a greater extent. On the other hand, high concentrations of tocopherols can exert a pro-oxidant activity34 and interact with other types of antioxidants in a complex manner, enhancing or attenuating each other’s effects. This could explain the observed changes in lycopene stability in GSOs enriched with the two carotenoid sources.
Conclusions
The results of this study indicate that functional seed oils containing lycopene as antioxidant ingredient can be easily prepared using a commercial tomato oleoresin as lycopene source. The stability of lycopene in the investigated oils is affected by both the temperature and the oil type. Stability can be easily quantified by estimating the half-life of the carotenoid under the conditions of interest. At 4 °C, the estimated half-life was about 300 days in GSO and 650 days in SSO.
Future studies should investigate the stability behavior of lycopene in other vegetable oils and the mechanisms responsible for stabilization. This would provide additional information for the optimal formulation of functional oil products to be used as dietary sources of lycopene.
Acknowledgements
This research was partially supported by funding from Sapienza University of Rome. The authors gratefully thank LycoRed Ltd. (Beer Sheva, Israel) for the kind gift of tomato oleoresin samples.
References
- Cámara, M.; de Cortes Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, R.M.; Manzoor, S.; Caceres, J.O; Stud. Nat. Prod. Chem. 2013, 40, 383–426.
CrossRef - Johnson, E.J.; Proc. Soc. Exp. Biol. Med. 1998, 218, 115–120.
CrossRef - Srivastava, S.; Srivastava, A.K.; J. Food Sci. Technol. 2015, 52, 41–53.
CrossRef - Palozza, P.; Parrone, N.; Simone, R.; Catalano, A.; Curr. Med. Chem. 2011, 18, 1846–1860.
CrossRef - Devaraj, S.; Mathur, S.; Basu, A.; Aung, H.H.; Vasu, V.T.; Meyers, S.; Jialal, I.; J. Am. Coll. Nutr. 2008, 27, 267–273.
CrossRef - Benakmoum, A.; Abbeddou, S.; Ammouche, A.; Kefalas, P.; Gerasopoulos, D.; Food Chem. 2008, 110, 684–690.
CrossRef - Zuorro, A.; Lavecchia, R.; Int. Rev. Chem. Eng. 2011, 3, 93–98.
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L.; Food. Bioprocess Technol. 2013, 6, 3499–3509.
CrossRef - Barros, H.D.F.Q.; Grimaldi, R.; Cabral, F.A.; J. Supercrit. Fluids. 2017, 120, 1–6.
CrossRef - Hackett, M.M.; Lee, J.H.; Francis, D., Schwartz, S.J.; J. Food Sci. 2004, 69, 536–541.
CrossRef - Nguyen, M.L.; Schwartz, S.J.; Exp. Biol. Med. 1998, 218, 101–105.
CrossRef - Shi, J.; Food Sci. Nutr. 2000, 40, 1–42.
- Caris-Veyrat, C.; Schmid, A.; Carail, M.; Bohm, V.; J. Agri. Food Chem. 2003, 51, 7318–7325.
CrossRef - Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J.; J. Med. Food 2005, 8, 413–422.
CrossRef - Shi, J.; Wu, Y.; Bryan, M.; Le Maguer, M.; Nutraceut. Food 2002, 7, 179–183.
- Lee, M.T.; Chen, B.H.; Food Chem. 2002,78, 425–432.
CrossRef - Ax, K.; Mayer-Miebach, E.; Link, B.; Schuchmann, H.; Schubert, H.; Eng. Life Sci. 2003, 4, 199–201.
CrossRef - Zuorro, A.; Sep. Purif. Technol. 2015, 152, 64–69.
CrossRef - Ramezan, Y.; Ghavami, M.; Bahmaei, M.; Givianrad, M.H.; Hemmasi, A.H.; Orient. J. Chem. 2015, 31, 1389–1394.
CrossRef - Leclercq, S.; Reineccius, G.A.; Milo, C.; J. Agric. Food Chem. 2007, 55, 9189–9194.
CrossRef - Codex Alimentarius. Codex standard for named vegetable oils, Codex Standard 210–1999, FAO and WHO, Rome, 2009.
- Rubalya, V.S.; Neelamegam, P.; Res. J. Chem. Environ. 2012, 16, 87–94.
- Wongnarat, C.; Srihanam, P.; Orient. J. Chem. 2017, 33, 113–121.
CrossRef - Wati, M.; Sheetal; Khabiruddin, M; Orient. J. Chem. 2017, 33, 1969–1975.
CrossRef - Seppanen, C.M.; Song, Q.H.; Csallany, A.S.; J. Am. Oil Chem. Soc. 2010, 87, 469–481.
CrossRef - Mortensen, A.; Skibsted, L.H.; Truscott, T.G.; Arch. Biochem. Biophys. 2001, 385, 13–19.
CrossRef - Krinsky, N.I.; Yeum, K.J.; Biochem. Biophys. Res. Commun. 2003, 305, 754–760.
CrossRef - Mortensen, A.; Skibsted, L.H.; Truscott, T.G.; Arch. Biochem. Biophys. 2001, 385, 13–19.
CrossRef - Al-Malki, A.L.; Moselhy, S.S.; Refai, M.Y.; Toxicol. Ind Health 2012, 28, 542–548.
CrossRef - Hazewindus, M.; Haenen, G.R.M.M.; Weseler, A.R.; Bast, A.; Food Chem. 2012, 132, 954–958.
CrossRef - Shi, J.; Le Maguer, M.; Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42.
CrossRef - Toor, R.K.; Savage, G.P.; Food Res. Int. 2005, 38, 487–494.
CrossRef - Kochhar, S.P.; Eur. J. Lipid Sci. Technol. 2000, 102, 552–559.
CrossRef - Shahidi, F.; Nahrung 2000, 44, 158−163.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









