Germanium Nanoparticles, Synthesis in Spark Discharge
Dmitry Mylnikov1 , Anna Lizunova1, Vladislav Borisov1, Sergey Paranin1,2 and Viсtor Ivanov1,2
, Anna Lizunova1, Vladislav Borisov1, Sergey Paranin1,2 and Viсtor Ivanov1,2
1Moscow Institute of Physics and Technology, 9, Institutsky per., Dolgoprudny, 141700, Russia.
2Institute of Electrophysics, Russian Academy of Sciences, Ural Branch, 106, Amundsen Street, Ekaterinburg, 620016, Russia.
Corresponding Author E-mail: mylnikov.da@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/340563
Article Received on : 19-06-2018
Article Accepted on : 20-08-2018
Article Published : 11 Oct 2018
In this paper we present a simple method of Ge nanoparticles synthesis in spark discharge generator (SDG) in pure argon flow. The sizes of the obtained primary particles are in the range of 5-15 nm and particles themselves are combined in agglomerates 80-200 nm in size. Transmission electron microscopy images as well as energy dispersive X-ray analysis and X-Ray diffraction analysis prove that the nanoparticles consist of crystalline germanium. Specific energy consumption of nanoparticle synthesis is 1,37 kW∙h/g.
KEYWORDS:Crystalline; Germanium; Nanoparticles; Spark Discharge Generator; Synthesis
Download this article as:| Copy the following to cite this article: Mylnikov D, Lizunova A, Borisov V, Paranin S, Ivanov V. Germanium Nanoparticles, Synthesis in Spark Discharge. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Mylnikov D, Lizunova A, Borisov V, Paranin S, Ivanov V. Germanium Nanoparticles, Synthesis in Spark Discharge. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50613 |
Introduction
Recent years have seen an increase in using various materials in the form of nanoparticles as well as their potential and functional applications in hi-tech fields. For example, the embedding of germanium nanoparticles into the oxide layer in the floating gate of the MOS memory devices gives a lower leakage current and a lower writing voltage. Such MOS devices with a nanoparticle embedded SiO2 gate dielectric have been studied both experimentally1–3 and theoretically.4 Also germanium is a promising material for replacing a carbon in the anode of lithium-ion batteries. It allows to increase the specific capacity of the battery anode by 5 times and the volume capacity by 10 times.5 Such batteries have been investigated both with pure Ge nanoparticles5–8 and GeO2 particles.9,10
The germanium aerosol produced in SDG can be used in aerosol printing to fabricate semiconductor elements, for example transistors. It can be done by simply replacing the silver electrodes with germanium ones in the existing setup.11
Also, the Ge nanoparticles demonstrate photoluminescent properties12 like quantum dots.
This paper is a continuation of our previous investigations13–15 of nanoparticle synthesis. In this work we have improved the gas discharge chamber by removing all plastic and fiberglass parts and silicone hoses. The new chamber has been made of stainless steel and glass. These changes have improved the purity of the neutral gas in the chamber and allowed us to obtain unoxidized Ge nanoparticles. Prior to our experiment, only synthesis of silicon nanoparticles out of other semiconductor materials has been demonstrated in spark discharge.16,17
Materials and Methods
In SDG, the capacitor storage is charged up to the breakdown voltage of the interelectrode gap and spark discharge occurs. During the discharge, a current of the order of hundreds of amperes flows through the gap. Due to the joule and radiation heating of the electrodes surface, the electrode material evaporates and subsequently condensates into the nanoparticles.
The basic scheme of the setup was similar to the one presented earlier.11 The interconnections were assembled from standard stainless steel vacuum fittings KF-16 and KF-25, and the synthesis chamber was tailored of heat-resistant glass. The volume was pumped to a pressure of 10 Pa, and then filled with the argon of purity 6.0. The synthesis was carried out in argon flow from a gas cylinder. The electrodes were clamped in collet-type holders, one of which was made movable for the gap distance adjustment. We used one pair of electrodes. The synthesis chamber was cooled outside by blowing air with a fan.
Gas was fed into the synthesis chamber through one of the electrodes having a tube-like shape. We will call it the anode because during the first half-cycle of oscillation, when the current amplitude is maximal, there was a positive voltage on it. The anode was a tantalum tube with outer diameter of 8 mm and inner diameter of 4 mm.
For cathode, we used a n-type doped single crystal germanium in the form of cylinder with a length of 30 mm and a diameter of 8 mm with polished ends. The specific resistance of this germanium was 0.005 Ohm∙cm, which corresponds to a doping concentration of 10^19 cm-3.18
The purpose of choosing different materials for cathode and anode was to obtain germanium nanoparticles using blanks of germanium rods that are easier to manufacture than rods with a hole. We have chosen tantalum as an anode material due to its refractoriness to obtain erosion predominantly from the germanium electrode, as the melting point of tantalum is 3017°C compared to 938°C for germanium.
The electrical high voltage circuit contained a voltage source and a capacitor storage was made of KVI-3 capacitors with a total capacity of 39 nF. The capacitor storage was charged to the voltage at which the gap breaks and damped oscillations occur in the circuit. After the breakdown, the voltage source disconnects for a while, then the next charging cycle begins. The frequency of charging the capacitors storage can be adjusted.
Nanoparticles aerosol passed through a nanofiber filter AFA-RMV-20, onto which they were precipitated for X-Ray diffraction analysis (XRD) on the Thermo Scientific ARL X’TRA X-Ray diffractometer equipped with a parabolic mirror (AXO Dresden) and a pinhole collimator.
For the transmission electron microscopy (TEM) analysis, a TEM grid was attached to the filter. After collecting the particles, it was conserved in hexane to prevent oxidation of nanoparticles during its extraction from the chamber, storage and transportation to the working volume of the TEM instrument. We used JEOL JEM2100 transmission electron microscope with energy dispersive X-ray (EDX) spectrometer X-MAX N OXFORD Instruments for the elemental analysis.
Mass output was determined by weighing the electrodes before and after the experiment on a Sartogosm CE-224 C balance.
The particle size distribution in the flow was measured using a TSI SMPS 3936 Aerosol spectrometer. In fact, these measurements give an equivalent size of the primary nanoparticles agglomerates. As the spectrometer was calibrated to measure aerosols in the air flow and nitrogen properties are close to the air ones, the flow of argon with aerosol was diluted 1:10 with nitrogen 6.0 before flowing to the spectrometer input. The path of the aerosol from the synthesis site to the entrance to the spectrometer was 70 cm.
Results and Discussion
Figure 1 depicts the results of agglomerates’ size distribution measurement made in aerosol flow downstream the synthesis chamber. Since nanoparticles produced in SDG tend to agglomerate and SMPS spectrometer uses an electrical mobility detection technique it shows size distribution of agglomerates and not the primary particles. The agglomerates size distribution mode is 120 nm. To reduce agglomeration one can use higher gas flow rates and shorten path from the site of synthesis to place of analysis. In our experiments with flow rate of 1 slpm and the path length between synthesis chamber and spectrometer of 70 cm the particles manage to agglomerate considerably.
TEM images show that the primary particles have a nearly spherical shape of 5-15 nm in diameter (fig. 2a,b). Almost all particles have a crystalline structure. Selected area electron diffraction pattern shows the characteristic rings of the germanium crystals (fig. 2c). Energy dispersive X-ray analysis (EDX) of primary particles indicates the presence of a small amount of oxygen (fig. 2d,e). When scanning along a particle, the intensity of germanium changes, and the oxygen intensity remains constant. This probably indicates that a very thin oxide film is present on the surface of the particles. The particles are united in agglomerates with dimensions of ~100 nm.
Figure 3a presents a photograph of the germanium particles deposited on the filter. The particles have a dark-brown color that does not change with time (over several days).
X-ray diffraction was measured for the particles in amount of several mg together with the filter on which they were deposited. Then the XRD pattern of the clean filter without particles was captured and subtracted from the first graph. The result is shown in the figure 3b. In X-ray diffraction patterns 3 characteristic peaks of germanium with a cubic lattice of the spatial group Fd3m are clearly visible, namely for planes (111), (220) and (311). No peaks of GeO or GeO2 was found. It means that powder consists of pure crystalline germanium particles. As the XRD analysis was carried out 24 hours after deposition of the particles on the filter and has not shown presence of germanium oxides, it may indicate the resistance to oxidation of germanium nanoparticles due to the formation of a very thin and strong oxide film on the surface.
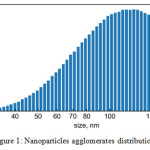 |
Figure 1: Nanoparticles agglomerates distribution. |
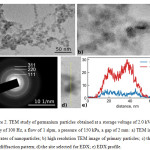 |
Figure 2: TEM study of germanium particles obtained at a storage voltage of 2.0 kV, a frequency of 100 Hz, a flow of 1 slpm, a pressure of 150 kPa, a gap of 2 mm: |
a) TEM image of agglomerates of nanoparticles; b) high resolution TEM image of primary particles; c) the electron diffraction pattern; d) the site selected for EDX; e) EDX profile.
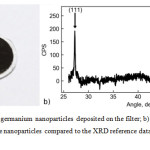 |
Figure 3a: Photo of germanium nanoparticles deposited on the filter; b) X-ray diffraction pattern of sample with Ge nano particles compared to the XRD reference data (AMCSD № 0009269). |
The mass output rate of germanium nanoparticles was 9.6 mg/h at a storage voltage of 2 kV, a discharge frequency of 160 Hz, a gas flow of 1 slpm, a pressure of 150 kPa, and a gap between electrodes of 2 mm. This corresponds to a specific energy consumption of 1,37 kW∙h/g. As the mass output rate is linear on pulse repetition frequency and spark energy,19 it can be further increased by increasing the pulse frequency and storage voltage. This is promising result for using SDG generated Ge nanoparticles in different applications. In this experiment we had to limit electrical power because the temperature of the glass synthesis chamber reached a critical value. The erosion of the tantalum electrode was 1.7 mg/h, which confirms our assumption that with tantalum anode and germanium cathode we will have mainly germanium particles in resulting powder composition.
Conclusion
We have shown a simple method of synthesizing germanium nanoparticles in SDG with a output rate of 9.6 mg/h, which can be even enhanced by increasing spark frequency and discharge voltage after organizing better cooling of the synthesis chamber.
The particles have a primary size of 5-15 nm and are combined in agglomerates, the X-ray analysis confirms that they consist of crystalline germanium.
The obtained germanium nanoparticles have a wide range of potential applications, including aerosol printing, lithium batteries, MOS memory and many others.
Acknowledgements
This work was supported by the Ministry of Education and Science of the Russian Federation (project № RFMEFI57517X0160).
References
- Kanoun, M.; Busseret, C.; Poncet, A.; Souifi, A.; Baron, T.; Gautier, E. Solid-State Electron. 2006, 50, 1310–1314.
CrossRef - King, Y.-C.; King, T.-J.; Hu, C. IEEE Trans. Electron Devices 2001, 48, 696–700.
CrossRef - Yang, M.; Chen, T. P.; Wong, J. I.; Liu, Y.; Tseng, A. A.; Fung, S. J. Nanosci. Nanotechnol. 2010, 10, 4517–4521.
CrossRef - Chakraborty, G.; Sengupta, A.; Requejo, F. G.; Sarkar, C. K. J. Appl. Phys. 2011, 109, 064504.
CrossRef - Li, W.; Sun, X.; Yu, Y. Small Methods 2017, 1.
- Bogart, T. D.; Chockla, A. M.; Korgel, B. A. Curr. Opin. Chem. Eng. 2013, 2, 286–293.
CrossRef - Lee, H.; Kim, M. G.; Choi, C. H.; Sun, Y.-K.; Yoon, C. S.; Cho, J. J. Phys. Chem. B 2005, 109, 20719–20723.
CrossRef - Park, M.-H.; Kim, K.; Kim, J.; Cho, J. Adv. Mater. 2010, 22, 415–418.
CrossRef - Seng, K. H.; Park, M.-H.; Guo, Z. P.; Liu, H. K.; Cho, J. Angew. Chem. 2012, 124, 5755–5759.
CrossRef - Seng, K. H.; Park, M.; Guo, Z. P.; Liu, H. K.; Cho, J. Nano Lett. 2013, 13, 1230–1236.
CrossRef - Efimov, A.; Potapov, G.; Nisan, A.; Urazov, M.; Ivanov, V. Orient. J. Chem. 2017, 33, 1047–1050.
CrossRef - Oku, T.; Nakayama, T.; Kuno, M.; Nozue, Y.; Wallenberg, L. R.; Niihara, K.; Suganuma, K. Mater. Sci. Eng. B 2000, 74, 242–247.
CrossRef - Efimov, A.; Volkov, I.; Varfolomeev, A.; Vasiliev, A.; Ivanov, V. Orient. J. Chem. 2016, 32, 2909–2913.
CrossRef - Ivanov, V. V.; Efimov, A. A.; Mylnikov, D. A.; Lizunova, A. A.; Bagazeev, A. V.; Beketov, I. V.; Shcherbinin, S. V. Tech. Phys. Lett. 2016, 42, 876–878.
CrossRef - Mylnikov, D. A.; Lizunova, A. A.; Efimov, A. A.; Ivanov, V. V. AIP Conf. Proc. 2017, 1858, 030003.
- Lee, D.; Lee, K.; Kim, D. S.; Lee, J.-K.; Park, S. J.; Choi, M. J. Aerosol Sci. 2017, 114, 139–145.
CrossRef - Vons, V. A.; de Smet, L. C.; Munao, D.; Evirgen, A.; Kelder, E. M.; Schmidt-Ott, A. J. Nanoparticle Res. 2011, 13, 4867–4879.
CrossRef - Thurber, W. R.; Mattis, R. L.; Liu, Y. M.; Filliben, J. J. J. Electrochem. Soc. 1980, 127, 2291–2294.
CrossRef - Tabrizi, N. S. Generation of nanoparticles by spark discharge, University of Tarbiat Modarres, Tehran University of Leeds, Leeds, 2009.

This work is licensed under a Creative Commons Attribution 4.0 International License.









