Functionalization of Cinnamaldehyde to Arylidene Barbituric Acid Catalyzed by Samarium(III) Chloride
Antonius Herry Cahyana , Bayu Ardiansah and Aisyah Nadila
, Bayu Ardiansah and Aisyah Nadila
Department of Chemistry, FMIPA, Universitas Indonesia, Depok, 16424, Indonesia.
Corresponding Author E-mail: herrykim@ui.ac.id
DOI : http://dx.doi.org/10.13005/ojc/340565
Article Received on : 16-08-2017
Article Accepted on : 22-08-2018
Article Published : 06 Sep 2018
Benzaldehyde and cinnamaldehyde were successfully condensed with barbituric acid using samarium(III) chloride in water for 2h at room temperature. The products were confirmed through FTIR, UV-Vis and GC-MS analysis.
KEYWORDS:Arylidene Barbituric Acid; Cinnamaldehyde; Condensation; Functionalization; Samarium(III) Chloride
Download this article as:| Copy the following to cite this article: Cahyana A. H, Ardiansah B, Nadila A. Functionalization of Cinnamaldehyde to Arylidene Barbituric Acid Catalyzed by Samarium(III) Chloride. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Cahyana A. H, Ardiansah B, Nadila A. Functionalization of Cinnamaldehyde to Arylidene Barbituric Acid Catalyzed by Samarium(III) Chloride. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=49480 |
Introduction
Cinnamaldehyde (C9H8O) is an aryl aldehyde and appears as yellow oily liquid.1 Naturally, it is the main constituent (60-65%) of leaf and bark extract of cinnamon, particularly in Indonesian (Cinnamomum burmannii) and Indian (Cinnamomum zeylanicum) cinnamon.2,3 This compound provides wide range of biological action, like anticancer,4 anti-inflammatory,5 antibacterial6 and antifungal7 properties. Effort to modify cinnamaldehyde into more useful products with attractive bioactivities has been shown in the last decade.8-10 The presence of α,β-unsaturated carbonyl group makes cinnamaldehyde as prominent precursor for synthesis of organic molecules with high complexity via various types of organic transformation.8-11
One of the key reactions which can be used to modify aryl aldehydes is Knoevenagel condensation. It is valuable reaction to form new C-C bond in the production of larger fine chemicals.12 This reaction involves the abstraction of proton from active methylene compound to give enolate ion then undergo nucleophilic attack to carbonyl group.12 In 2006, Li et al. synthesized 5-arylidenes barbituric acid via Knoevenagel condensation using grinding method.13 Various yields of desirable product were found from 35-96% with 1 to 15 min grinding time and 120 min laying time.13 Khurana and Vij (2010) performed Knoevenagel condensation of aromatic aldehydes with barbituric acids and 2-thiobarbituric acids using nickel nanoparticles with medium to good yield of isolated products.14 Other investigation in Knoevenagel condensation using both of homogeneous and heterogeneous catalysts were found in the literature, but some of them were accompanied with limitations, such as in catalyst preparation or the use of harmful solvent.8,12,15-17 Therefore, the search for efficient method or catalyst for Knoevenagel condensation is still become a major challenge.
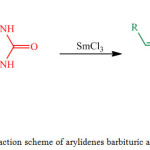 |
Figure 1: Reaction scheme of arylidenes barbituric acid synthesis. |
In this research, we synthesize the arylidene derivatives of barbituric acid using samarium (III) catalyst and characterize the products using some analytical instruments such as FTIR, UV-Vis, and GCMS.
Materials And Methods
General
All chemicals were analytical grade and obtained from commercial suppliers. FTIR spectra were recorded on Shimadzu Prestige-21 FTIR spectrophotometer. Ultraviolet-visible absorptions were screened using Shimadzu 2450 UV-Vis spectrophotometer. GC-MS analyses were done by Agilent 7890A-5975C.
Synthesis of Arylidenes Barbituric Acid
Aldehyde (1 mmol), barbituric acid (2 mmol), samarium(III) chloride (10 wt.%) in water (2 mL) were stirred for 2 h at room temperature in an Erlenmeyer flask. The reaction was monitored using thin layer chromatography. The product formed will precipitate. After completion, solid product was filtered and recrystallized from hot ethanol.
Compound 3a, 5-benzylidenepyrimidine-2,4,6(1H,3H,5H)-trione has physical and spectral data as follows: pale yellow solid, m.p. 261-262oC (lit. 271oC18); 61% yield; Rf 0.75 (ethyl acetate : ethanol 7:3) IR (KBr, cm-1): 3629 (N-H), 3025 (C-H sp2), 1693 (C=O), 1386 (C-N), 697 (mono-substituted benzene). UV-Vis (nm): 230, 258. GC (min): 13.51. MS (m/z): 216 (M+), 215, 172, 129, 102.
Compound 3b, (E)-5-(3-phenylallylidene)pyrimidine-2,4,6(1H,3H,5H)-trione has physical and spectral data as follows: yellow solid, m.p. 260-261oC (lit. 270oC18); 68% yield; Rf 0.58 (ethyl acetate : ethanol 7:3) IR (KBr, cm-1): 3629 (N-H), 3088 (C-H sp2), 1701 (C=O), 1559 (C=C stretch), 1221 (C-N), 669 (mono-substituted benzene). UV-Vis (nm): 372. GC (min): 15.61. MS (m/z): 242 (M+), 171, 155, 128, 127.
Results and Discussion
The FTIR, UV-Vis, and GC-MS spectra of compound 3a are presented in the Figure 2. In FTIR spectrum, the peaks at 3629, 3025, and 1693 cm-1 are corresponding to the secondary N-H stretching, C-H sp2 stretching, and C=O stretching, respectively. Additional peak appeared at 1386 cm-1 due to C-N stretching and peak at 697 cm-1 indicated the compound 3a as mono-substituted benzene. Compound 3a has maximum absorption at 258 nm. The structure confirmation was determined by GC-MS. From GC-MS analysis, compound 3a is the most abundant with retention time at 13.51 min and M+ value of 216.
 |
Figure 2: FTIR, UV-Vis, and GC-MS spectra of compound 3a. |
Figure 3 showed the FTIR, UV-Vis, and GC-MS spectra of compound 3b. The major absorption peaks in FTIR spectrum are observed at 3629, 3088, and 1701 cm-1 which attributed to the secondary N-H stretching, C-H sp2 stretching, and C=O stretching vibration, respectively. The peaks at 1559 and 1221 cm-1 are correspond to the C=C and C-N stretching vibration, respectively. It is indicated the successful adduct between cinnamaldehyde with barbituric acid to give compound 3b. The maximum absorption of 3b is occurred at 372 nm. Analysis using GC-MS revealed that compound 3b is formed with molecular cation value of 242. It is in good agreement with our previous work, when the formation of cinnamaldehyde-barbituric acid adduct was conducted using citric acid supported on Fe3O4 as catalyst.11
 |
Figure 3: FTIR, UV-Vis, and GC-MS spectra of compound 3b. |
The possible mechanism of Knoevenagel condensation between aryl aldehyde and barbituric acid is depicted in Figure 4. The first step is keto-enol tautomerism of barbituric acid to give structure in enol form. Then a physical interaction occurs between SmCl3 as Lewis acid catalyst with oxygen atom in carbonyl group, which activate the carbon atom. In the second step, the activated carbonyl group is attacked by an enol to produce compound that contains new carbon-carbon bond. The last step is dehydration process to give final product. The product obtained in medium yield probably due to competition between acid base interaction of the catalyst with lone pair electrons in oxygen or nitrogen atom.
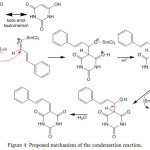 |
Figure 4: Proposed mechanism of the condensation reaction. |
Conclusion
The synthesis arylidenes barbituric acid using samarium(III) chloride as Lewis acid catalyst has been done in mild condition. Compound 3a and 3b are formed in medium yield, 61 and 68%, respectively. The successive formation of these compounds was confirmed by some analytical instruments including FTIR, UV-Vis, and GC-MS. This research highlights an environment-friendly synthesis of organic compound promoted by simple catalyst.
Acknowledgement
This work was financially supported by The Directorate General of Higher Education (DIKTI), Ministry of Research, Technology and Higher Education, Republic of Indonesia through Penelitian Unggulan Perguruan Tinggi (PUPT) 2017 Research Grant.
References
- Shreaz, S.; Wani, W. A.; Behbehani, J. M.; Raja, V.; Irshad, Md.; Karched, M.; Ali, I.; Siddiqi, W. A.; Hun, L. T. Fitoterapia. 2016, 112, 116-131.
CrossRef - Wang, R.; Wang, R.; Yang, B. Innov. Food Sci. Emerg. Tech. 2009, 10, 289-292.
CrossRef - Lin, L.-T.; Wu, S.-J.; Lin, C.-C. J. Trad. Complement. Med. 2013, 3(4), 227-233.
- Gan, F. F.; Chua, Y. S.; Scarmagnani, S.; Palaniappan, P.; Franks, M.; Poobalasingam, T.; Bradshaw, T. D.; Westwell, A. D.; Hagen, T. Biochem. Biophys. Res. Commun. 2009, 387, 741-747.
CrossRef - Ahn, S.; Kim, E.; Lee, K.; Lee, D.-C. Int. Immunopharmacol. 2016, 38, 342-348.
CrossRef - Lee, H. S.; Ahn, Y. J. J. Agric. Food Chem. 1998, 46, 8-12.
CrossRef - Aziz, A. N.; Ibrahim, H.; Rosmy Syamsir, D.; Mohtar, M.; Vejayan, J.; Awang, K. J. Etnopharmacol. 2013, 145, 798-802.
CrossRef - Rajput, J. K.; Kaur, G. Chinese J. Catal. 2013, 34, 1697-1704.
CrossRef - Cahyana, A. H.; Pratiwi, D.; Ardiansah, B. Rasayan J. Chem. 2016, 9(4), 896-902.
- Cahyana, A. H.; Nadila, A.; Ardiansah, B. AIP Conf. Proc. 2017, 1862, Article ID 030097. DOI: 10.1063/1.4991201.
CrossRef - Cahyana, A. H.; Pratiwi, D.; Ardiansah, B. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 188, Article ID 012008. DOI: 10.1088/1757-899X/188/1/012008.
CrossRef - Tangale, N. P.; Sonar, S. K.; Niphadkar, P. S.; Joshi, P. N. J. Ind. Eng. Chem. 2016, 40, 128-136.
CrossRef - Li, J.-T.; Dai, H.-G.; Liu, D.; Li, T.-S. Synth. Commun. 2006, 36, 789-794.
CrossRef - Khurana, J. M.; Vij, K. Catal. Lett. 2010, 138, 104-110.
CrossRef - Wang, C.; Ma, J.-J.; Zhou, X.; Zang, X.-H.; Wang, Z.; Gao, Y.-J.; Cui, P.-L. Synth. Commun. 2005, 35, 2759-2764.
CrossRef - Sakthivel, B.; Dhakshinamoorthy, A. J. Coll. Interf. Sci. 2017, 485, 75-80.
CrossRef - Rana, S.; Jonnalagadda, S. B. Catal. Commun. 2017, 92, 31-34.
CrossRef - Alcerreca, G.; Sanabria, R.; Miranda, R.; Arroyo, G.; Tamariz, J.; Delgado, F. Synth. Commun. 2000, 30(7), 1295-1301.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









