Electronic Structure Mechanism of Axial Ligands on Itinerant Electrons and Negative Magnetoresistance in Axially-Ligated Iron(III) Phthalocyanine Molecular Conductors
Eiza Shimizu1 and Derrick Ethelbhert Yu1,2
1Department of Chemistry, College of Science, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
2Materials Science and Nanotechnology Unit, Center for Natural Sciences and Environmental Research, De La Salle University, 2401 Taft Avenue, Manila, Philippines.
Corresponding Author E-mail: derrick.yu@dlsu.edu.ph
DOI : http://dx.doi.org/10.13005/ojc/340511
Article Received on : 15-08-2018
Article Accepted on : 12-10-2018
Article Published : 18 Oct 2018
Partially-oxidized Iron(III) phthalocyanine (FeIIIPc) with axial CN or Br ligands are molecular conductors with giant negative magnetoresistance. Electron conduction occurs via intermolecular overlapping of Pc p-orbitals, while negative magnetoresistance is brought about by intramolecular interaction between Fe-d and Pc-p orbitals. Aside from permitting slip-stacked solid-state arrangement, axial ligands can further enhance the p-d interaction of FeIII(Pc) depending on the strength of ligand field energies that proportionally leads to larger negative magnetoresistance. However, the strong ligand field of CN results in conductivity reduction due to the p-accepting nature of the ligand which enhances electron gradient in the oxidized Fe3+, thereby localizing itinerant electrons in Pc, as evidenced by charge transfers between Fe-d and CN-p orbitals. In contrast, the p-donating nature of Br ligands complements the electron deficiency of Fe3+, resulting in the delocalization of itinerant electrons in the Pc system, thus creating a highly conducting molecular system with giant negative magnetoresistance.
KEYWORDS:Axially-ligated iron(III) phthalocyanine; Giant negative magnetoresistance; molecular conductor; pi-d interaction
Download this article as:| Copy the following to cite this article: Shimizu E, Yu D. E. Electronic Structure Mechanism of Axial Ligands on Itinerant Electrons and Negative Magnetoresistance in Axially-Ligated Iron(III) Phthalocyanine Molecular Conductors. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Shimizu E, Yu D. E. Electronic Structure Mechanism of Axial Ligands on Itinerant Electrons and Negative Magnetoresistance in Axially-Ligated Iron(III) Phthalocyanine Molecular Conductors. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=51103 |
Introduction
Phthalocyanine (Pc) is a planar molecule composed of four circular N-linked isoindole rings forming a fully-conjugated 18 p-electron system – ideal structural and chemical characteristics as building blocks of functional materials. In recent years, molecular engineering has been employed to the Pc moiety to utilize its potential solid-state applications, particularly as organic conductors. Octahedral-coordinating central metal such as Co3+(d6) and Fe3+(d5) are incorporated to Pc to enable the attachment of linear axial ligands such as CN, thereby forming slip-stacked solid-state arrangement between adjacent CoIII/FeIII(Pc)(CN)2 units, resulting in the intermolecular overlapping of p-orbitals between Pc rings (HOMO), and thus generating electron conduction path/band upon partial-oxidization of the Pc system (electron hole transport).1
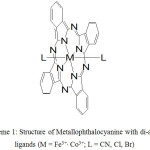 |
Scheme 1: Structure of Metallophthalocyanine with di-axial ligands (M = Fe3+, Co3+; L = CN, Cl, Br). |
Axial ligands can further modify the properties of the metal phthalocyanine system. For CoIII(Pc)L2 system, electrical conductivity are correlated to the effectiveness of intermolecular Pc p-p orbital overlap emanating from the bulkiness/steric effect of the axial ligands which are in the order: L = CN > Cl > Br.2
The incorporation of magnetic Fe3+ (s = 1/2) central metal produces highly conducting FeIII(Pc)L2 (L = CN, Cl, Br) series with anisotropic giant negative magnetoresistance (GNMR). The GNMR is brought about by the interaction between the d-orbital of Fe3+ and the p-orbital of the Pc moiety. Interestingly, it was observed that the intensity of p-d interaction is affected and can be modulated by the varying magnitudes of axial ligand field energy.3
Axial ligands affect the electronic structure of the FeIII(Pc) system resulting in the modulation of orbital interactions. Ab initio calculations derived the differences (proximity) between the p and d orbital energies which correspond to the intensity of p-d interaction as 8.5450 eV and 7.8655 eV for L = Br and CN, respectively4. It was established that the axial ligand correspondingly lifts Fe-d orbitals nearer to Pc-p orbitals depending on its field energy, thereby intensifying p-d interaction. Moreover, the extent of the p-orbitals of the axial ligands results into stronger d-d interactions.5,6 Thus, higher axial ligand field energy equates to stronger p-d orbital interaction.
The intensity of the p-d interaction has a direct correlation effect on the GNMR of the FeIII(Pc)L2 system. At magnetic field of 15 Tesla, FeIII(Pc)(CN)2 and FeIII(Pc)Br2 have exhibited 93% and 67% reduction of electrical resistivity (GNMR), respectively. However, the intensity of GNMR in FeIII(Pc)L2 (L = CN > Br) have an inverse effect on the normal electrical conductivity (at ambient temperature and magnetic field) of the FeIII(Pc)L2 series which resulted in the order: L = CN < Br, wherein a 2-order electrical conductivity decrease in FeIII(Pc)(CN)2 attributed to p-d interaction, and no decrease in the electron transport of FeIII(Pc)Br2 were observed,even as the Br-ligated species also has a relatively strong p-d interaction as demonstrated by its GNMR profile.3
For years, there was a prevailing concept that the existence of p-d interaction in the FeIII(Pc)L2 system will always consequently equate to electron localization (decrease in conductivity). However, an absence of electron localization was observed in the FeIII(Pc)Br2 system, where its electrical conductivity is in the same order as its Co homologue, CoIII(Pc)Br2, in which there is no p-d interaction. In terms of molecular structure, the electronic conduction band width of FeIII(Pc)L2 stood unaffected by the p-d and GNMR interplay, as it remained to be based on the effectiveness of the intermolecular Pc p-p orbital overlap brought about by the steric effect of the axial ligands.7 Thus, the origin of the p-d interaction – electron localization – GNMR interplay phenomenon in the FeIII(Pc)L2 system may be traced to its electronic structure. And the elucidation of the electronic structure mechanism of FeIII(Pc)L2 may provide new prospects in the design of functional highly conducting molecular systems with GNMR.
Materials and Methods
Tetraphenylphosphonium (TPP) salts of axially-ligated metallophthalocyanines: TPP[FeIII(Pc)(CN)2]2, TPP[FeIII(Pc)Br2]2 and TPP[CoIII(Pc)(CN)2]2 were synthesized using previously reported methods2,3. For TPP[CoIII(Pc)(CN)2]2 and TPP[FeIII(Pc)(CN)2]2: 30 mg CoII(Pc)/FeII(Pc) and 60 mg tetraphenylphosphosnium iodide (TPPI) was dissolved in 40 mL propionitrile (CH3CH2CN) in a two-compartment electrochemical cell. Applied current was set at 10μA. Partially-oxidized TPP[CoIII(Pc)(CN)2]2 and TPP[FeIII(Pc)(CN)2]2 took 6-8 weeks to crystallize with a yield of about 10-15%. For TPP [FeIII(Pc)Br2]2: 30 mg FeII (Pc) and 60 mg TPPBr were suspended in 40 mL 1:3 dimethylformamide (DMF) : acetone solvent system and subsequently mixed via a sonicator. A current of 5 μA was then applied to the system. Electrocrystallization of the partially-oxidized TPP[Fe(Pc)Br2]2 takes 2-3 weeks to accomplish. The product conversion is between 60-70%.
UV-Vis solution absorption spectra of TPP[FeIII(Pc)(CN)2]2, TPP[FeIII(Pc)Br2]2 and TPP[CoIII(Pc)(CN)2]2 in Dimethylformamide were measured from 13000 cm-1 to 30000 cm-1 using a JASCO Ubest V570 spectrophotometer.
Results and Discussion
The electronic absorption spectra is an effective method to experimentally describe the electronic structure and corresponding orbital interactions of the FeIII(Pc)L2, which is a unique single molecule p-d system.
![Figure 1: UV-Vis spectra of TPP[FeIII(Pc)(CN)2]2, TPP[FeIII(Pc)Br2]2 and TPP[CoIII(Pc)(CN)2]2.](http://www.orientjchem.org/wp-content/uploads/2018/10/Vol34No5_Ele_Eiz_fig1-150x150.jpg) |
Figure 1: UV-Vis spectra of TPP[FeIII(Pc)(CN)2]2, TPP[FeIII(Pc)Br2]2 and TPP[CoIII(Pc)(CN)2]2. |
Figure 1 displays the UV-Vis absorption spectra of TPP[FeIII(Pc)(CN)2]2, TPP[FeIII(Pc)Br2]2 and TPP[CoIII(Pc)(CN)2]2 from 13000 to 30000 cm-1.The characteristic intra-Pc ring p → p* transitions: Q (1a1u(p) → 1eg(p*)) at around 15000 cm-1 (665 nm), and B/Soret: (1a2u(p) → 1eg(p*)) at around 27500 cm-1 (365 nm) were observed for all species. Also, for the FeIII(Pc)(CN)2 species, two metal to axial ligand charge transfers (MLCT) were detected: MLCT1 = (eg(dp) → 1b1u(p*)) at around 18500 cm-1 (540 nm), and MLCT2 = (eg(dp) → 1b2u(p*)) at around 25000 cm-1 (400 nm).8
The charge transfer (CT) between Fe3+ and CN ligands in FeIII(Pc)(CN)2 and the absence of CT between Fe3+ and Br ligands in FeIII(Pc)Br2, despite having p-d interactions, points to the axial ligands as the origin of the electron localization/delocalization mechanism in the FeIII(Pc)L2 system. That is, the oxidized state of Fe3+ (vacancy in eg(dp)) enables the attraction of electron density from electron-rich Pc (Fe-dyz to Pc-pz), while axial CN which is a strong p-acceptor further intensifies the withdrawal of electron density from Fe3+ (to CN px-orbital), thereby resulting to the localization of itinerant electrons in Pc. In contrast, the strong p-donor character of Br appears to compensate for the electron deficiency of Fe3+, thereby favoring electron delocalization that results in the easing of electron transport on the part of the Pc moiety. Moreover, the absence of the MLCT peaks in CoIII(Pc)(CN)2 system (Figure 1) further ascertains that the nature of MLCT1 and MLCT2 are induced by the p-d electron interplay in the FeIII(Pc)(CN)2 species.
Conclusion
The strong p-d interaction in FeIII(Pc)L2 system always result in GNMR. Axial ligands enhance the p-d interaction depending on their field energy. It is established that the p-d interaction in FeIII(Pc)L2 does not always result in electron localization. Electron localization is not dependent on the p-d but on the electronic character of the axial ligands.
Employing axial ligands that are p-donor (weak field energies) by nature will still significantly enhance the p-d interaction of FeIII(Pc) to produce GNMR with the absence electron localization. p-donor ligand such as Br compensates for the electron deficiency of the oxidized Fe3+, thereby easing itinerant electrons in Pc to favor electron delocalization. Thus, it is possible to create a highly conducting magnetic molecular system with GNMR such as FeIII(Pc)Br2 which has strong p-d interaction and unhampered electron transport.
Acknowledgement
The authors are grateful for the financial support from the Department of Science and Technology – Science Education Institute (DOST-SEI) of the Philippine Government.
References
- Inabe, T; Tajima, H. Phthalocyanines – Versatile Components of Molecular Conductors. Chem. Rev. 2004, 104, 5503-5534.
CrossRef - Yu, D.E.C.; Imai, H.; Ushio, M.; Takeda, S.; Naito, T.; Inabe, T. One-Step Synthesis of Partially-Oxidized Salts of Cobalt(III) Phthalocyanine with Axial Ligands. Chem. Lett. 2006, 35, 602-603.
CrossRef - Yu, D.E.C.; Matsuda, M.; Kikuchi, A.; Taketsugu, T.; Naito, T.; Inabe, T. Variable Magnetotransport Properties in the TPP[Fe(Pc)L2]2 System (TPP = Tetraphenyl- phosphonium, Pc = Phthalocyaninato, L = CN, Cl, Br). J. Mater. Chem. 2009, 19, 718-723.
CrossRef - Yu, D.E.C.; Kikuchi, A.; Taketsugu, T.; Inabe, T. Crystal structure of Ruthenium Phthalocyanine with di-axial monoatomic ligand: Bis(triphenylphosphine)iminium dichloro(phthalocyaninato(2-))ruthenium(III). J. Chem. 2013, 486318(1-6).
- Inoue, M.; Torizuka, K.; Tajima, H.; Matsuda, M.; Yu, D.E.C.; Naito, T,; Inabe, T.; Hanasaki, N. Magnetic Torque Measurements of TPP[Fe(Pc)Br2]2. Physica B 2010, 405, S331-333.
CrossRef - Torizuka, K.; Tajima, H.; Inoue, M.; Hanasaki, N.; Matsuda, M.; Yu, D.E.C.; Naito, T.; Inabe, T. Magnetic Torque Experiments on TPP[Fe(Pc)L2]2: Antiferromagnetic Short-Range Ordering of d Electrons, Antiferromagnetic Ordering of π Electrons, and Anisotropy Energy. J. Phys. Soc. Jpn. 2013, 82, 034719(1-14).
- Yu, D.E.C.; Matsuda, M.; Tajima, H.; Naito, T.; Inabe, T. Stable p-p dependent electron conduction band of TPP[M(Pc)L2]2 molecular conductors. Dalton Trans. 2011, 40, 2283-2288.
CrossRef - Ough, E.A.; Stillman, M.J. Analysis of the Absorption and Magnetic Circular Dichroism Spectra of Low Spin (S=1/2) Iron (III) Phthalocyanine. Inorg. Chem. 1995, 34, 4317-4325.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









