Efficacy of Corrosion Inhibitive Properties of Gum Exudates of Azadirachta Indica on Carbon Steel in 1N Hydrochloric Acid
M. Malarvizhi1 and J. Mallika2
1Department of Chemistry, Sri GVG Visalakshi College for Women, Udumalpet-642128, Tamil Nadu, India.
2Department of Chemistry, PSG College of Arts and Science, Coimbatore-641014, Tamil Nadu, India.
Corresponding Author E-mail: jmchempsg2016@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/340534
Article Received on : 27-02-2018
Article Accepted on : 08-08-2018
Article Published : 12 Sep 2018
Corrosion mitigation of carbon steel by neem gum (gum exudates of Azadirachta indica) was investigated in 1N HCl medium. Effect of temperature and immersion period towards the mitigation process is studied usingweight loss method.Electrochemical studies combined with surface analysis indicate that corrosion reaction is inhibited by adsorption of GAI. Adsorption process is consistent with Langmuir isotherm. The inhibitor behaves as mixed type for carbon steel corrosion in 1N HCl. Surface morphology studies reveal adsorption on the metal surface.
KEYWORDS:Corrosion Inhibition; Carbon Steel; Gum Exudates of Azadirachta Indica; HCl; SEM
Download this article as:| Copy the following to cite this article: Malarvizhi M, Mallika J. Efficacy of Corrosion Inhibitive Properties of Gum Exudates of Azadirachta Indica on Carbon Steel in 1N Hydrochloric Acid. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Malarvizhi M, Mallika J. Efficacy of Corrosion Inhibitive Properties of Gum Exudates of Azadirachta Indica on Carbon Steel in 1N Hydrochloric Acid. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=49618 |
Introduction
Carbon steel (CS) is extensively used in constructions as well as for industrial applications.1,2 Under different working environments, CS is liable to undergo corrosion. Though many ways are available for corrosion inhibition of metals, use of inhibitors to arrest the corrosion process is considered the best.3 Many synthetic organic compounds are reported as corrosion inhibitors.4 Due to their environment and health constraint5, use of natural plant products as corrosion inhibitors are extensively studied.6 Since gum exudates of plants and trees have the potential to act as corrosion inhibitors, many naturally occurring gum exudates were studied as corrosion inhibitors.7-9 The complexity of constituents, tendency to form complexes with metal ions and eco-friendly nature make the gum exudates good corrosion inhibitors.10 A survey of literature showed that the neem gum ie., gum exudates of Azadirachta indica (GAI) has not been employed towards corrosion mitigation of carbon steel in hydrochloric acid medium. Azadirachta indica gum is reported to contain D- glucose, D-glucoronic acid, L-arabinose, L-fucose, mannose, xylose, rhamnose, D- glucosamine, aldobiuronic acid, serine, threonine and aspartic acid.11,12 In addition to that it also found to contain organic fatty acids.13 Considering the advantages of gum exudates as corrosion inhibitors and the presence of complex organic constituents in GAI, the current investigation is aimed to examine the possibility of gum exudates of Azadirachta indica as a CS corrosion inhibitor in HCl solution. The inhibition efficiency has been tested using the following methods: Mass loss, Polarization and AC impedance methods. The effect of temperature (303- 328±1 K) and immersion time (1, 2, 4, 6 , 8 and 10 h) on the corrosion performance of CS was also analyzed. The carbon steel surface in the absence and presence of inhibitor has been examined by using scanning electron microscopy.
Materials and Method
Neem gum obtained from local area was purified. Analar grade sulphuric acid solution was used as corrosive medium. All solutions were prepared using double distilled water. The Carbon steel coupons of composition 0.37 % C,0.680 % Mn, 0.230 % Si, 0.160 % Cu, 0.077 % Cr, 0.059 % Ni, 0.015 % S, 0.011 % Ti, 0.009 % Co, remaining Fe having dimensions of 25 x 10 x 1 mm was used to carry out weight loss experiments on the basis of ASTM practice standard G-3114.
Following equations (1),(2) and (3) were used for calculation purpose.
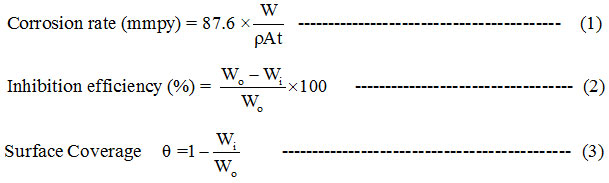
where W, ρ , A , t , Wi and Wo represents weight loss in g, density of CS coupon in g cm˗3, area of the coupon in cm2, time of immersion in h, loss of weight in inhibited and free acid solutions respectively. The electrochemical tests were carried out using a conventional three electrode system at room temperature. Along with the CS working electrode, saturated calomel electrode and platinum electrodes were used as reference and counter electrodes respectively. Scan rate in the case of polarization studies was 1mV s-1 and the potential range studied was +200 to −200mV. Frequency range of 10 KHz to 0.01 Hz was set for impedance studies while 10 mV amplitude AC signals were employed for OCP with prior immersion of 30 minutes. Zview software was used to obtain impedance data as Nyquist plots from which the Rct values were found. JEOL-JSM-35-CF SEM instrument was employed to analyze the exterior of inhibited and uninhibited carbon steel specimens.
Results and Discussion
Effect of Inhibitor Concentration
Effect of GAI on the corrosion process of CS in 1N HCl medium studied at 303±1 K using gravimetric measurements for different immersion periods up to 10 hours. Table 1 shows the corrosion rate (CR), inhibition efficiency (I E) and surface coverage (θ). Analysis of Table 1 indicates that there was a gradual decrease in corrosion rate with increase in GAI concentration showing the concentration dependence of corrosion rates. Beyond 80 ppm concentration, no considerable inhibition performance of GAI was observed which specifies the optimum concentration as 80 ppm. The gum showed the maximum inhibition efficiency of 83.04% for 1 h immersion period at an optimum concentration of 80ppm. Primarily the protection act was due to GAI accumulation on to the CS surface which reduces the acid contact of the metal. The high outcome for relatively longer immersion period could be due to insoluble filmy coat or corrosion product formed on CS surface15 which prevents the penetration of Cl− ions of aggressive medium and inward diffusion of oxygen, thereby decreasing the corrosion rate.16
Table 1: Effect of different concentrations of GAI on CS in 1N HCl for various immersion periods at 303K.
| C | 1 hr | 2 hrs | 4 hrs | ||||||
| ppm | CR | I E | θ | CR | I E | θ | CR | I E | θ |
| mmpy | (%) | mmpy | (%) | mmpy | (%) | ||||
| Blank | 46.11 | 49.27 | 52.21 | ||||||
| 5 | 32.1 | 30.39 | 0.3039 | 31.87 | 35.32 | 0.3532 | 31.08 | 40.48 | 0.4048 |
| 10 | 28.03 | 39.22 | 0.3922 | 26.44 | 46.33 | 0.4633 | 25.65 | 50.87 | 0.5087 |
| 20 | 23.51 | 49.02 | 0.4902 | 21.47 | 56.42 | 0.5642 | 21.36 | 59.09 | 0.5909 |
| 40 | 18.08 | 60.78 | 0.6078 | 16.95 | 65.6 | 0.656 | 15.93 | 69.48 | 0.6948 |
| 60 | 14.47 | 68.63 | 0.6863 | 13.11 | 73.39 | 0.7339 | 11.98 | 77.06 | 0.7706 |
| 80 | 13.56 | 70.59 | 0.7059 | 9.49 | 80.73 | 0.8073 | 7.8 | 85.06 | 0.8506 |
| 100 | 14.92 | 67.65 | 0.6765 | 9.27 | 81.19 | 0.8119 | 7.57 | 85.5 | 0.855 |
| 120 | 13.11 | 71.57 | 0.7157 | 9.04 | 81.65 | 0.8165 | 7.23 | 86.15 | 0.8615 |
| C | 6hrs | 8hrs | 10 hrs | ||||||
| ppm | CR | I E | θ | CR | I E | θ | CR | I E | θ |
| mmpy | (%) | mmpy | (%) | mmpy | (%) | ||||
| Blank | 55.07 | 58.26 | 60.03 | ||||||
| 5 | 28.4 | 48.43 | 0.4843 | 32.04 | 45 | 0.45 | 34.67 | 42.24 | 0.4224 |
| 10 | 25.16 | 54.31 | 0.5431 | 29.61 | 49.18 | 0.4918 | 31.82 | 46.99 | 0.4699 |
| 20 | 20.87 | 62.11 | 0.6211 | 24.18 | 58.49 | 0.5849 | 31.1 | 48.19 | 0.4819 |
| 40 | 14.09 | 74.42 | 0.7442 | 21.53 | 63.05 | 0.6305 | 28.07 | 53.24 | 0.5324 |
| 60 | 11.38 | 79.34 | 0.7934 | 19.49 | 66.54 | 0.6654 | 27.3 | 54.52 | 0.5452 |
| 80 | 9.34 | 83.04 | 0.8304 | 18.36 | 68.48 | 0.6848 | 25.09 | 58.21 | 0.5821 |
| 100 | 9.42 | 82.9 | 0.829 | 15.93 | 72.65 | 0.7265 | 22.78 | 62.05 | 0.6205 |
| 120 | 9.57 | 82.63 | 0.8263 | 14.92 | 74.39 | 0.7439 | 22.92 | 61.82 | 0.6182 |
Effect of Immersion Period
Stability of GAI on CS surface is evaluated by weight loss measurements in the inhibited and uninhibited solutions for 1–10 hours of immersion time at 303K and given in Table 1. On close inspection, it can be understood that efficiency of the inhibitor increased with immersion period revealing the protective layer formation on CS due to strong adsorption of GAI. There was a linear relationship of weight loss with immersion period. As the immersion period and the GAI concentration increases, a chemical mode of adsorption was expected.17 The inhibitor efficiency increases gradually attaining maximum for 5h immersion time suggesting that the stability and effectiveness of the inhibitor withstands over a period of 6 hrs. After an immersion period of 6 h, it can be noticed that the inhibition efficiency slowly started to decrease.
Thermodynamic Considerations
Influence of temperature on inhibition behavior of GAI was studied at the temperatures 303K, 313 K and 323 K and the obtained results were shown in Table 2. With increase in temperature, corrosion rate increases in the presence and absence of GAI due to increase in the kinetic energy of the reacting entities. Corrosion rate at any given concentration decreases for the inhibited acid solution compared to the uninhibited solution in all temperature range studied was as a result of mitigating effect of the gum on the corrosion reaction of CS. With increase in temperature, a linear but a slight increase in inhibition efficiency was observed. At all temperatures studied, inhibition performance lowered or remained similar after 80 ppm GAI. The adsorption process may be either physical or chemical adsorption.18 The fact is decided by nature and structure of the inhibitor, pH and temperature of the system and character and charge of the metal.18
Table 2: Corrosion parameters for different concentrations of GAI on CS in 1N HCl at 303K, 313K and 323 K.
| C | 303K | 313K | 323K | ||||||
| ppm | CR mmpy | I E | θ | CR mmpy | I E | θ | CR mmpy | I E | θ |
| (%) | (%) | (%) | |||||||
| Blank | 46.11 | 150.08 | 265.8 | ||||||
| 5 | 32.1 | 30.39 | 0.3039 | 101.26 | 32.53 | 0.3253 | 174.94 | 34.18 | 0.3418 |
| 10 | 28.03 | 39.22 | 0.3922 | 86.79 | 42.17 | 0.4217 | 150.98 | 43.2 | 0.432 |
| 20 | 23.51 | 49.02 | 0.4902 | 74.59 | 50.3 | 0.503 | 125.22 | 52.89 | 0.5289 |
| 40 | 18.08 | 60.78 | 0.6078 | 56.51 | 62.35 | 0.6235 | 95.38 | 64.12 | 0.6412 |
| 60 | 14.47 | 68.63 | 0.6863 | 43.85 | 70.78 | 0.7078 | 75.49 | 71.6 | 0.716 |
| 80 | 13.56 | 70.59 | 0.7059 | 41.14 | 72.59 | 0.7259 | 65.55 | 75.34 | 0.7534 |
| 100 | 14.92 | 67.65 | 0.6765 | 40.68 | 72.89 | 0.7289 | 60.57 | 77.21 | 0.7721 |
| 120 | 13.11 | 71.57 | 0.7157 | 41.59 | 72.29 | 0.7229 | 63.29 | 76.19 | 0.7619 |
Based on the following Arrhenius equation,19 the apparent activation energy (E*a) was evaluated using frequency factor A from the equation (4). A plot (Fig. 1) was made between log CR and 1/T resulting in the slope of – E*a/2.303 R and intercept A from which Ea and A were obtained .The enthalpy and entropy parameters ∆S* and ∆H* were obtained using equation 5. A transition state curve between log(CR/T) versus 1/This plot. This plot figure. 2 a straight line with slope (−∆H*/2.303 R) and intercept log (R/Nh) + ∆S*/2.303 R.
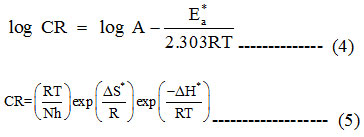
Table 3: Corrosion parameters for CS in 1N HCl in the absence and presence of GAI in the temperature range of 303K-323K.
|
Concentration (ppm) |
Ea (kJmol-1) |
∆H* (kJmol-1) |
Ea – ∆H* (kJmol-1) |
∆S* (Jmol-1k-1) |
|
Blank |
72.83 |
70.18 |
2.66 |
19.17 |
|
5 |
70.50 |
67.85 |
2.66 |
8.46 |
|
10 |
70.02 |
67.36 |
2.66 |
5.69 |
|
20 |
69.55 |
66.89 |
2.66 |
2.77 |
|
40 |
69.14 |
66.48 |
2.66 |
-0.79 |
|
60 |
68.70 |
66.04 |
2.66 |
-4.18 |
|
80 |
65.51 |
62.85 |
2.66 |
-15.14 |
|
100 |
58.26 |
55.61 |
2.66 |
-38.30 |
|
120 |
65.46 |
62.80 |
2.66 |
-15.46 |
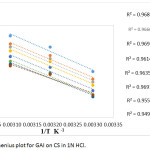 |
Figure 1: Arrhenius plot for GAI on CS in 1N HCl. |
Table 3 displays the obtained values for Ea , ∆H* and ∆S*. Activation energy for free acid solution is 72.83 KJmol-1 which decreases to 58.26KJmol-1 in the presence of GAI. This indicates that GAI is adsorbed chemically which in turn reduces the corrosion rate.20-22 For any given concentration Ea value is larger than ∆H* value. Decrease in ∆H* value for th inhibited solution compared to blank also supports the chemical nature of adsorption. Endothermic nature of CS dissolution is confirmed by positive values of ∆H*.23, 24 The free acid solution has a ∆S* value of 19.17 JK-1mol-1 which decreases up to a maximum of -38.30 JK-1mol-1 in the presence of GAI which actually signifies the conversion of reactants to activated complexes.25
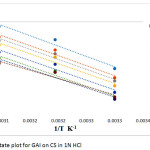 |
Figure 2: Transition State plot for GAI on CS in 1N HCl |
Adsorption Isotherm
Generally the adsorption process of organic molecules over the metal surface depends on chemical composition of the organic compound, temperature, and electrochemical potential on metal.26, 27 Adsorption isotherms signifies the mechanistic path of adsorption. In the present study the Langmuir isotherm model was found as best fitting one.Langmuir isotherm equation relates equilibrium constant Kads and concentration as in equation (6) and ΔGads, free energy of adsorption is calculated from the equation (7).
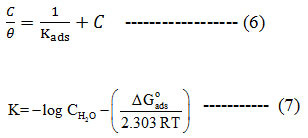
Langmuir adsorption plots obtained at different temperatures with various concentrations of GAI is shown in Figure 3. Strong adsorption is evident from the strong correlation coefficients R2 > 0.99 at all temperatures. Calculated ΔGads is presented in Table 4. Slope values also confirms the validity of the Langmuir model. Negative ΔGads values shows that the adsorption process is spontaneous.28 Generally, ΔGads around -20 kJ/mol is suggestive of physisorption and around -40 kJ/mol is said to be chemisorption.29 So it is implicit that ΔGads values presented in table 4 is reflective of physical nature of adsorption. But the Ea values reveal that a little of chemical bonding may also be involved in the adsorption process.
Table 4: Thermodynamic adsorption parameters for the different concentration of GAI on CS in 1N HCl in the temperature range of 303-323K.
|
Temperature K |
Slope n |
Equilibrium constant (Kads) mol -1 |
-ΔG°ads kJmol-1 |
||
|
303 |
1.3 |
76.527 |
-21.28 |
||
|
313 |
1.2 |
93.520 |
-21.47 |
||
|
323 |
1.2 |
112.138 |
-21.39 |
||
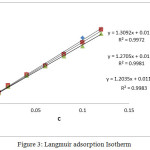 |
Figure 3: Langmuir adsorption Isotherm. |
Electrochemical Considerations Electrochemical Polarization Studies
Polarization potential curves applied for the assessment of corrosion mitigation effect of GAI on CS specimen in 1N HCl is presented in Figure 4. By usual extrapolation method, corrosion current Icorr and potential Ecorr is evaluated and the inhibition efficiency was assessed by equation (8).
![]()
From figure 4, it was clear that corrosion rate decreases in the presence of GAI as a consequence of production of lower current densities by both anionic dissolution of metals and cationic hydrogen evolution reaction. Change in the tafel constants prove that GAI affects both anodic and cathodic reaction. Table 5 shows the electrochemical parameters Icorr and Ecorr IE(%), Tafel constants bα and bc. From the table, it is evident that there is decrease in corrosion rate in GAI inhibited solution compared to blank solution. Decrease in Icorr value shows the retardation of electrochemical reactions. In addition absence of significant change in Ecorr values is indication of mixed mode type.30 The inhibition efficiencies obtained in this method follows the similar pattern observed in gravimetric experiments.
Table 5: Polarization data of CS in 1N HCl in the presence and absence of GAI.
| Concentration (ppm) | ICorr(mA cm-2) | Ecorr (Mv /SCE) | bα(mV dec-1) | bc(mV dec-1) | IE (%) |
| Blank | 763 | – 471.28 | 84.4 | 133 | – |
| 40 | 312 | – 433.20 | 69 | 150 | 59.11 |
| 60 | 268 | -436.26 | 72 | 138 | 64.88 |
| 100 | 234 | -434.91 | 77 | 140 | 69.33 |
AC Impedance Spectroscopy Studies
In order to study the adsorption nature of CS in 1N HCl at room temperature, impedence responses were recorded for free acid and inhibited test solutions which is shown in the figure 5. This shows Nyquist plots increasing in diameter of semi-circles with GAI concentration which is indicative of the fact that GAI adsorption on to the metal hinders the corrosion process. Difference in the shape of the semicircles can be explained with reference to non-homogeneity of the surface and roughness of the metal.31 Charge transfer resistance, double layer capacitance and inhibition efficiency values were presented in table 6.On evaluation of table 6 shows, Cdl values were found to decrease with increase in Rct.This may be attributed to the fact that the adsorbed film deposited by the green inhibitor on the surface of the electrode. The Cdl valueof uninhibited solution is 45.3 X 10-3 μF cm-2 which decreases to 20.8 X 10-3μF cm-2 on addition of 100 ppm GAI. Thus there was a decrease in Cdl values with increase in concentration. This can be accounted by any of the two factors: lowering of dielectric constant and increase of thickness in double layer capacitance.32 Thus electrical capacity is decreased through the displacement of ions and water molecules on the surface interface.33 Shapes of the semicircle with and without inhibitor look alike indicating an unaltered corrosion mechanism on the addition of GAI.
Table 6: AC Impedance data of CS in 1N HCl in the presence and absence of GAI.
| Concentration(ppm) | Rct (Ω cm-2 ) | Cdl ( μF cm-2) * 10-3 | IE (%) |
| Blank | 12.1 | 45.1 | – |
| 40 | 51.3 | 29.7 | 76.41 |
| 60 | 84.8 | 23.1 | 85.73 |
| 100 | 101.3 | 20.8 | 88.05 |
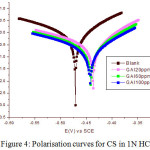 |
Figure 4: Polarisation curves for CS in 1N HCl. Figure: 5 Nyquist plots of CS in 1N HCl. |
 |
Figure 5: Nyquist plots of CS in 1N HCl. |
Scanning Electron Microscopy
To validate film formation, SEM images of CS coupons exposed to inhibited and uninhibited solutions were documented. Figures 6a, 6b and 6c show freshly recorded SEM images of polished CS surface, CS surface exposed to uninhibited solution and CS surface exposed to 100 ppm of GAI respectively.
 |
Figure 6: SEM photographs of CS sample. |
Figure 6a shows plain clear surface, while Figure 6b depicts severely corroded surface due to acid attack and Figure 6c represents smooth surface with some scattered pits. This changes in the surface of the CS specimen is a supportive feature of formation of adsorbed film. Thus the addition of GAI to 1N HCl has reduced the corrosion of carbon steel specimen significantly.
Conclusion
GAI shows significant corrosion inhibition for CS in hydrochloric acid medium. With increase in temperature, inhibition efficiency increases. Inhibition process takes place through adsorption mechanism. Langmuir is the best fit isotherm. Spontaneity of the adsorption process is shown by thermodynamic parameters. The polarization measurements is the evidence for GAI to be a mixed mode inhibitor. The SEM analysis supports protective film formation on CS.
Acknowledgement
The authors are thankful to PSG College of Arts and Science, Coimbatore and Sri GVG Visalakshi College for Women, Udumalpet for supporting with the lab facilities.
References
- Deyab, M.A. Corros. Sci. 2007, 49, 2315–2328.
CrossRef - Hamdy, A.; Nour Sh. El-Gendy. Egypt. J. Petr. 2013, 22,17–25.
- Trabanelli, G.; Corr. 1991, 47, 410-419.
CrossRef - Abhay Singh. SSRG Inter. J. App.Chem. 2017, 5,21-25.
- Ostovari, A.; Hoseinieh, M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corro. Sci. 2009, 51, 1935-1949
CrossRef - Janaina Cardozo da Rocha.; José Antônio da Cunha Ponciano Gomes.; Eliane D’Elia. Corros. Sci. 2010, 52, 2341–2348.
- Umoren, S.A.; Obot, I. B.; Ebenso, E.E. J. Chem. 2008, 5(2), 355-364.
- Buchweishaija, J.; Mhinzi, G.S. Port. Electrochim. Acta. 2008, 26, 257-265.
CrossRef - Abdallah, M. Port. Electrochim. Acta. 2004, 22, 161-175.
CrossRef - Eddy, N.O.; Ameh, P.; Gimba, C.E.; and Ebenso, E.E. Int. J.Electrochem. Sci. 2012, 7, 7425- 7439.
- Allman, M.A.; Pena M.M.; Peng. D. Eur. J. Clin. Nutr. 1995, 49, 169-178.
- Anderson, D.M.W.; Hendrie, A. Carbohyd. Res. 1971, 20, 259-268
CrossRef - Mukerjee, S.; Srivastava, H.C. J. Am. Chem. Soc. 1955, 77,422- 423.
CrossRef - ASTM practice standard G-31.; Standard practice for laboratory immersion corrosion testing of metals.; ASTM International. 2004.
- Granese, S. L., Rosales, B. M.; Ociede, C., and Zerbino J.O. Corros Sci. 1992, 33, 1439 – 1453.
CrossRef - Al-Fozan, S.A.; Malik, A. U. Desalination. 2008, 228, 61-67.
CrossRef - Trabanelli, G., Mansfeld, F. (Ed.), Corrosion Inhibitors, Chemical Industries: Corrosion Mechanism,Chapter 3,vol. 28, 1987, pp. 119–163.
- Blaedel, W. J.; Meloche, V.W.; Elementary Quantitative Analysis; Theory and Practice, Second Edition, Harper & Row, Publishers, Incorporated, 49 East 33rd Street, New York 16, New York. 1963, 684-698.
- Rani, P. D.; Selvaraj, S. Journal of Phytology. 2010, 2 (11), 58-64.
- Umoren, S A.; Ogbobe, O.; Ebenso E E. J Appl Polym Sci. 2007, 105 , 3363-3370.
CrossRef - Orubite, K .O.; Oforka, N.C. J Mater. Lett. 2004, 58,1768-1772.
CrossRef - El-Etre, A .Y. J. Appl. Surf. Sci. 2006, 252 , 8521-8525.
CrossRef - Szauer, T.; Brandt, A. Electrochim. Acta, 1981, 26, 1209-1217.
CrossRef - Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.; Hammouti, S.; Bouyanzer, B. J. Electrochem. Sci. 2010, 5 ,1060-1069.
- Saliyan, V.R.; Adhikari, A.V. Bull. Mater. Sci. 2007, 31, 699-711.
CrossRef - Gerengi, H.; Goksu, H.; Slepski, P. Mater. Res. 2014 , 17(1) 255-264.
CrossRef - Oguzie, E. E. Corros. Sci., 2007, 49(3) 1527-1539.
CrossRef - Ali, S. A.; Saeed, M. T.; Rahman, S. U. Corros. Sci. 2003, 45, 253-266.
CrossRef - Ali, S.A.; El-Shareef, A.M.; Al-Ghamdi, R.F.; Saeed, M.T. Corros. Sci. 2005 , 47, 2659-2678.
CrossRef - Muralidharan, S.; Quraishi, M.; Iyer, S. V. K. Corros. Sci., 1995 , 37,1739 -1750.
CrossRef - Bentiss, F.; Jama, C.; Mernari, B. Corr. Sci. 2009,8, 1628-1635.
CrossRef - Outirite, M.; Lagrenee, M.; Lebrini, M.; Traisnel, M., Jama, C.; Vezin, H.; Bentiss, F. Electrochim. Acta. 2010, 55, 1670-1681.
CrossRef - Lagrenée, M.; Mernari B., Bouanis, M.; Traisnel, M., Bentiss, F.Corros. Sci. 2002, 44, 573-588.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









