An Innovative Application of Nano-Technology in the Production of Hybrid Inorganic-Organic Coatings of Crude Oil Tanks
Kauther Elhabeeb Belgacem1,2, Hala Mohamed Abo-Dief1,3 and Khamael Mohammed Abualnaja1
and Khamael Mohammed Abualnaja1
1Department of Chemistry, Faculty of Science, Al-Taif University, KSA.
2University of Bizert. Tunisia laboratory Lacresne.
3Egyptian Petroleum Research Institute Nasr City.
Corresponding Author E-mail: Mohamed.hala91@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/340503
Article Received on : 06-06-2018
Article Accepted on : 11-09-2018
Article Published : 15 Oct 2018
Three corroded mediums that found in the crude oil have been applied to carbon steel specimens. The effect of the corrosion mediums concentration, application periods and times investigated. The Gravimetric method showed that sodium thio sulfate has the highest corrosion effect at all corrosion test parameters followed by sodium sulfate and sodium carbonate respectively. Each of Nano zinc oxide, Nano cotton cellulose and Nano banana cellulose protective films inhibit sodium thio sulfate corrosion effect. Anti-corrosion behaviour of the formed Nano films studied using gravimetric method (weight loss method), electrochemical method (potentiodynamic polarization) and scanning electron microscope (SEM) techniques. The Nano zinc oxide heated at 700°C shown to have better inhibition efficiency followed by Nano zinc oxide heated at 600 OC, Nano cotton cellulose and Nano banana cellulose respectively. The potentiodynamic polarization measurements showed Nano zinc oxide heated at 700°C, Nano cotton cellulose, and Nano banana cellulose the acted as mixed-type inhibitors with predominantly cathodic effectiveness. SEM techniques supported the achievement of corrosion inhibition with the presence of inhibitors.
KEYWORDS:Potentiodynamic Polarization and Gravimetric Methods; Nano Cotton and Banana Cellulose; Nano Polyvinyl Alcohol; Sodium Thio Sulfate; Sodium Sulfate; Sodium Carbonate; SEM Images
Download this article as:| Copy the following to cite this article: Belgacem K. E, Abo-Dief H. M, Abualnaja K. M. An Innovative Application of Nano-Technology in the Production of Hybrid Inorganic-Organic Coatings of Crude Oil Tanks. Orient J Chem 2018;34(5). |
| Copy the following to cite this URL: Belgacem K. E, Abo-Dief H. M, Abualnaja K. M. An Innovative Application of Nano-Technology in the Production of Hybrid Inorganic-Organic Coatings of Crude Oil Tanks. Orient J Chem 2018;34(5). Available from: http://www.orientjchem.org/?p=50786 |
Introduction
Recently Nanotechnology application gained momentum, and environmental impact. Due to their extremely fine grain size and high grain boundary volume fraction Nano composites are known.1-4 Nano technology used in agricultural systems, biomedicine, environmental engineering, safety and security, water resources, energy conversion, and numerous other areas.5-9 Abo-Dief, Mostafa, Alzahrani, and Mohamed10 coated stainless steel oil pipes with chitosan (CH), Nano iron oxide (NFE) and Nano activated carbon (NAC). Abo-Dief, Ebrahim, Altalhi, and Mohamed,11 Haniffa, Chin, Abdullah, Poh, and Chuah,12 and Schincario13 used epoxies (polymers) for the best concrete quality. Bianco, Tosco, and Sethi14 used two carbon based NPs and single layer graphene oxide (SLGO). Luo, Wang, Wei, Xiao, and Ni,15 considered Nano-fluids have good properties of radiation absorption and heat transfer. Matteo, Candido, Vera, and Francesca16 concluded that Nanotech successfully used in drilling muds for the past 50 years. Walsh, Leon, Bavykin, Low, Wang, and Larson,16 investigated nanostructured metallic coatings including Nano crystalline, and Nano composite types.
Protective painting systems include a primer and topcoat.17 Saji18 deduced that for many years, coatings must have steel structures corrosion protection principal element. Drozdz, Hawkins, Clark, Surratt, Kingsley, Palutke, and Dean19 detected the polarization technique parameters effect on the oil pipelines corrosion. Galvanized steel with Nano composite has advantages in comparison with both the ZnO and PPy.20-23 The oxidation/corrosion of different metallic substrates improved using organic–inorganic hybrid coatings.24-25 Al-Naamani, Dobretsov, Dutta, and Burgess26 used (chitosan-ZnO) nanoparticle hybrid coatings and their antifouling activity tested. Abo-Dief, Morsi, and Mohamed,27 and Abo-Dief, Al-Ghamdi, Al-Zahrani, and Mohamed28 showed that addition of Nano Zn in the deposition process increases the corrosion resistance. Abo-Dief29 used positron annihilation, in detecting Zn, ZnO2 and Zn-ZSM-5 Nano powders properties. Abualnaja, and Abo-Dief,30 synthesed silver nanoparticles by different types of plants and Vera, Schrebler, Cury, Del Rı´o, Romero31 evaluated the corrosion protection of carbon steel using synthesed polyaniline and poly(ortho-methoxyaniline) (Poma). Abo-Dief, Emam, Abualnaja, and Mohamed32 producing the biodiesel from corn waste frying oil and five fresh vegetable oils (corn, rapeseed, sunflower, soybean and palm). Sherif, Saheb, El-Zatahry, kenawyand, and Alkaraki33 concluded that PVA and PVC coatings decrease the corrosion currents and corrosion rate of carbon steel. Pujari, S; Ramakrishna, A; and Kumar34 discussed the uses and applications of jute and banana fiber composites. The present work aimed at determining the Poly vinyl alcohol (PVA), Nano Banana and cotton cellulose and Nano Zinc oxide coated carbon steel corrosion efficiency against one of three aggressive media; sodium thio sulfate, sodium sulfate and sodium carbonate. The effect of the corrosion test periods, temperature and corrosion media concentrations carried out and investigated.
Experimental
Material Preparation
Test specimens prepared by polishing, rinsing with distilled water, degreasing in ethanol, and drying at hot air. Carbon steel specimens corrosion test specimens performed with a rectangular form of 2.0 cm × 1.0 cm × 0.2 cm at an immersion time of; 24 h, 48 h, 72 h and 96 h at 25°C, 60°C and 100°C. After immersion period, the specimens cleaned according and reweighed to 10-4 g for determining the weight loss. Three solutions; sodium thio sulfate, sodium sulfate and sodium carbonate of; 0.01 M, 0.03 M and 0.05 M concentrations used at 100 ml basic oil.
Preparation of Coating Material
Banana fruit and cotton fibers were purchased from local market and prepared as Nano cellulose. The concentration of both Nano materials mixed with water as; 1Wcellulose : 10Wwater. Polyvinil Alcohol is purchased and used as a coating medium. Zinc oxide is also purchased from the local market and heat treated at 600°C and 700°C and cooled in furnace and used as a corrosion medium.
Measurements
Gravimetric Measurements
After immersion period in the corrosion medium, the specimens cleaned and reweighed using a balance scale to 10-4 g for determining the weight loss. At each case, triplicate specimens used to get the mean value of the weight loss.
Electrochemical Measurements
The specimens of the higher corrosion medium subjected to the electrochemical measurements. The overall current density values, I, considered as the sum of two contributions, anodic and cathodic current Ia and Ic, respectively. The corrosion inhibition efficiency evaluated from the corrosion current densities values using the relationship (1):
IE = [(Io corr – Icorr) / Io corr] X 100 (1)
where Icorr and Io corr are the corrosion current densities values with and without inhibitor, respectively.35 The surface coverage (θ) obtained from polarization curves for various concentrations of inhibitor using the following equation (2):
θ = 1 – (Icorr / Io corr) (2)
where Icorr, and Io corrare the corrosion current densities values with and without inhibitor, respectively.35
Electrochemical Studies
Electrochemical studies carried using advanced corrosion measurement instrument. Three electrodes of carbon steel 1cm X 1cm X0.5cm as working electrode connected to anode, platinum as counter electrode connected to cathode and a reference electrode Ag/ AgCl used.
Potentiodynamic Polarization Measurement (TAFEL)
The TAFEL polarization curves obtained by scanning the electrode potential with respect to Ecorr with a scanning rate of 1 mVs-1. The linear segments of the anodic and cathodic curves extrapolated to obtain the corrosion current densities Icorr. Scanning Electron Microscope (Carl Zeiss Supra-55 SEM) used to monitor the surface morphological changes.
Results and Discussions
Weight loss Results
Effect of Corrosion Medium Concentration
Fig. 1 illustrated the effect of the three corrosion mediums concentrations of 0.01M (a), 0.03M (b) and 0.05M (c) on the carbon steel corrosion at different test periods and 25°C. The results reveal that the amount of weight loss of carbon steel increases with the corrosion medium concentrations. This is due to the increment of the attacking action of the corrosion medium and the weakling effect of the carbon steel metal. The figure showed that sodium thio sulfate has the highest corrosion effect followed by sodium sulfate and sodium carbonate respectively. Also, the figure showed a corrosion effect of the basic oil due to the corrosion constituents that usually found in the crude oil. There is a weight loss increment related to the sodium carbonate corrosion medium due to the sticking of both the sodium carbonate particles with the loosed carbon steel particles on the carbon steel corroded specimen.
 |
Figure 1: Variation of corrosion medium concentration with the weight loss of the carbon steel specimens at various test periods at room temperature. |
Effect of Test Period
Fig. 2 illustrated the Variation of test period with the weight loss of the carbon steel specimens at various concentrations at 60°C. it is clear that as the test period increases, the weight loss increases for all types of the corrosion mediums due to the increment application period of the carbon steel specimen to the attacking corrosion mediums that increases the weakness of the steel with time. As observed in 3.1.1. The sodium thio sulfate corrosion trend is found higher followed by sodium sulfate and sodium carbonate respectively.
 |
Figure 2: Variation of test period with the weight loss of the carbon steel specimens at various concentrations at 60°C. |
Effect of Test Temperature
Fig. 3 showed that as the test temperature increases, the weight loss increases for all types of the corrosion mediums due to the weakening of the carbon steel specimen which facilitate the attacking corrosion mediums. As observed in 3.1.1. The sodium thio sulfate corrosion trend is found higher followed by sodium sulfate and sodium carbonate respectively.
Electrochemical Results
Potentiodynamic polarization curves of carbon steel specimens immersed in the sodium thio sulfate the highest corrosion medium without and with Nano Polyvinyl-Alcohol (FE/PVL) zinc oxide at 600°C (FE 600ZnO), Nano banana cellulose (FE coat B), Nano cotton cellulose (FE coat C) and Nano zinc oxide at 700°C (Fe 700 ZnO) coating mediums are given Figure 4. The current corrosion density (Icorr), corrosion potential (V), inhibition efficiency (E%) and surface coverage values (θ) are given in Fig. 4. Fig. 4.a showed that carbon steel coated with Nano ZnO at 700°C has the lowest current corrosion density followed by carbon steel coated with Nano ZnO at 600°C, Nano cotton cellulose, Nano banana cellulose, and Polyvinyl-Alcohol respectively with (Icorr) values of; 0.19, 1.79, 5.5, 13.8 and 17.2 mA respectively. So, Nano ZnO at 700°C corrosion inhibitor has the highest corrosion resistance compared to the other corrosion inhibitors used. Also, it is clear that as the temperature increases, the inhibition effect of the coating increases in agreement with,36-38 where the Icorr value decrement means the inhibitor concentration increment. The decrease in Icorr value indicates that decrease in flow of electrons, i.e., corrosion is supressed on the specimen.
Fig. 4.b showed that carbon steel coated with Nano ZnO at 700°C has the lowest corrosion potential (Vcorr) followed by Nano banana cellulose, Nano cotton cellulose, carbon steel coated with Polyvinyl-Alcohol, and Nano ZnO at 600°C respectively with (Vcorr) values of; 1.01, 1.07, 1.09, 717.0 and 911.0 mV respectively. So, Nano ZnO at 700°C corrosion inhibitor has the lowest corrosion potential. As the Inhibitor classified as cathodic or anodic according to Vcorr value displacement from the control is greater than 85 mV. If the value is less than 85 mV, the inhibitor considered to be mixed type inhibitor. So, the Nano ZnO at 700°C, Nano banana cellulose, and Nano cotton cellulose inhibitors considered as mixed inhibitor and effective towards cathodic inhibition inagrement with.39
Fig. 4.c showed that carbon steel coated with Nano ZnO at 700°C has the highest Inhibition efficiency (E%) by carbon steel coated with Nano ZnO at 600°C, Nano cotton cellulose, Nano banana cellulose, and Polyvinyl-Alcohol respectively with (E%)values of; 99.5%, 95%, 84.9%, 62.2% and 52.0% respectively. Comparing the inhibition efficiency of Nano ZnO at 700°C and Nano ZnO at 600°C indicated that the inhibition efficiency is temperature-dependent in agreement with.37,39
Fig. 4.d showed that carbon steel coated with Nano ZnO at 700°C has the highest surface coverage (θ) followed by carbon steel coated with Nano ZnO at 600°C, Nano cotton cellulose, Nano banana cellulose, and Polyvinyl-Alcohol respectively with (θ)values of; 0.995, 0.9, 0.849, 0.622 and 0.52 respectively. Comparing the Inhibition efficiency of Nano ZnO at 700°C and Nano ZnO at 600°C indicated that the surface coverage is temperature-dependent in agreement with.36-39 Since the surface coverage increases with temperature related to the change in the nature of the adsorption mode; the inhibitor is being physically adsorbed at lower temperatures, while Physisorption is favored by increasing of temperature. Thus, at high surface coverage, diffusion through the surface layer containing the inhibitor and corrosion products became the rate-determining step of the metal dissolution process.37
Tafel Polarization Curves
The potentiodynamic polarization measurement allowed for the study of anticorrosion behaviour via anodic and cathodic polarizations. The measurements recorded and analysed as TAFEL plots shown in Figure 5 with the electrochemical parameters presented in Fig. 4. Fig. 5 illustrated the Tafel curves of Nano coatings. The corrosion potential is indicated by the intersection of the slopes of the cathodic and anodic branches of the curve. Figs. 5.a to 5.f showed the samples corrosion potential using various coating types. In agreement with the potentiodynamic test variables represented in Figure 4, the test specimen containing coated with ZnO at 700°C has the lowest corrosion effect than the other coating mediums. ZnO inhibitor at 700°C, banana and cotton has Ecorr value displacement less than 85 mV. The result implies that the inhibitor used is mixed inhibitor and effective towards cathodic inhibition. Maximum efficiency found to be 99.95% with ZnO coating at 700°C.
Surface Examination
Carbon steel surface before subjected to 0.05M sodium thio sulfate shown in Fig. 6.a. Carbon steel coated with Nano PVL, Nano cotton cellulose, Nano banana cellulose, Nano ZnO at 600°C and Nano ZnO at 700°C subjected to 0.05M sodium thio sulfate shown in Figs. 6.b to 6.e. The SEM results showed that in the absence of inhibitor, more oxidized product in the metal surface with more damages. Where in the presence of inhibitor, there is little corrosion takes place in the surface due to its binding to the metal which visible in the SEM images. ZnO coating at 700°C showed the best surface coverage.
 |
Figure 4: Potentiodynamic polarization test values for all types of coating mediums. |
 |
Figure 5: Cyclic voltametry Tafel plots of carbon steel corroded at 0.05M sodium thio sulfate with (a) no coating at 100°C, (b) coated with PVA, (c) coated with cotton, (d) coated with ZnO at 600°C, (e) ZnO 700°C and (f) coated with banana. |
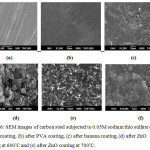 |
Figure 6: SEM images of carbon steel subjected to 0.05M sodium thio sulfate (a) before coating, (b) after PVA coating, (c) coated with cotton, (d) coated with ZnO at 600OC, (e) ZnO 700OC and (f) coated with banana. |
Conclusion
The Following Conclusions Obtained
As the corrosion medium concentration, test temperature and test periods increases, the corrosion effect increases.
Sodium thio sulfate has the highest corrosion effect followed by sodium sulfate and sodium carbonate respectively.
The Nano zinc oxide heated at 700°C shown to have better inhibition efficiency followed by Nano zinc oxide heated at 600°C, Nano cotton cellulose , Nano banana cellulose and Nano polyvinyl alcohol respectively.
Potentiodynamic polarization parameters (Icorr, Vcorr, θ. and E %) show that the used inhibitors adsorbed on carbon steel surface.
Good agreement observed between electrochemical curves and scanning electron microscopy images.
Polarization study reveals that Nano-zinc oxide heated at 700°C, Nano cellulose cotton, and Nano cellulose banana systems formulation are mixed type of inhibitor.
Acknowledgements
Authors are thankful to Al-Taif University for sponsoring and supporting this research under the project No (1-438-5622) and enabling us to develop this work.
References
- Abo-Dief, H.; Belgacem K, and Mohamed, A; RJPBCS, 8(4), 2017, 52-60.
- Javed, Mohammed; IJSRD, Vol. 2, Issue 11, 2015, 8-12.
- Pandey, R; Krishna, S; Rana, J; and Hazarika, N; IJTRE, 3, 10, 2016, 3087-3090.
- Wansah, J; Udounwa, A; Ahmed, A; Essiett, A; and Jackson, E; Proceedings of the 1st African Int. Conf./Workshop on Applications of Nanotechnology to Energy, Health and Environment, UNN, pp. 103-110, March 2014, 23 – 29.
- Orabi, R; Abo-Dief, H; Altalhi, A; and Mohamed, A; RJPBCS, 7, 2016, 815- 824.
- Manjunatha, S; Biradar, D; And Aladakatti, Y; J. Farm Sci., 29(1), 2016, 1-13.
- Adeleye, A; Conway, J; Garner, K; Huang, Y; Su, ; and Keller, Y; Chemical Engineering Journal, 286, 2016.
- Ahmed, Z; Bilal, K; Khan, A; and Riaz, N; IJCEM, 1, 12, March 2015, 11-58.
- Kunduru, K; Nazarkovsky, M; Farah, S; Pawar, R; Basu, A; Domb, A; Elsevier Inc, 2017, 33-74.
- Abo-Dief, H; Mostafa, N; Alzahrani, E; and Mohamed, A; (RJPBCS), Issue 7 (4), pp. 2999-3007, July-August, 2016.
- Abo-Dief, H; Ebrahim, F; Altalhi, A; and Mohamed, A; IJAST, 5, 2015, 43-52.
- Haniffa, M; Chin; Abdullah, L; Poh, S; and Chuah, C; Polymers, 8(7), 246; 2016, 1-12.
- Schincario, S.; Ph.D., Universita’ Degli Studi Di Padova Dipartimento, 2016.
- Bianco, C, Tosco, T; and Sethi, R; Contaminant Hydrology J., 93, 2016, 10–20.
- Luo, Z; Wang, C; Wei, W; Xiao, G; and Ni, M; International Journal of Heat and Mass Transfer, 75, 2014, 262–271.
CrossRef - Matteo, C; Candido, P; Vera, R; and Francesca, V; AJAS, 9 (6), 2012, 784-793.
CrossRef - Peng, H; Text Book, 2010, 333-387.
- Saji, V; Recent Patents on Corrosion Science, 2, 2010, 6-12.
CrossRef - Drozdz, S; Hawkins, T; Clark, L; Surratt, M; Kingsley, J; Palutke, K; and Dean, J; US Army Crops, 2011, 1-127.
- Amanzadeha, H; Yaminia, Y; and Moradi, M; Analytica Chimica Acta, 884, 2015, 52–60.
CrossRef - Walzade, S; and Tarannum, N; IRJET, Volume: 03 Issue: 08, 2016, 1277-1281.
- Cocuzza, M; Pirri, F; Rocca, V; and Verga, F; Politecnico di Torino, The Offshore Mediterranean Conference and Exhibition in Ravenna, Italy, 2011, 1-17.
- Umoren, S; and Solomon, M; The Open Materials Science J., 2014, 8, 39-54.
CrossRef - Xie, G; Luo, P; Deng, M; and Wang, Z; J. of NanomaterialsVolume, 2015, 1-8.
- Figueira, R; Silva, C; and Pereira, E; J. Coat. Technol. Res., 12 (1), 2015, 1–35.
CrossRef - Al-Naamani, L; Dobretsov, S; Dutta, J; and Burgess, J; Chemosphere 168, 2017, 408-417.
CrossRef - Abo-Dief, H; Morsi, S; and Mohamed, A; IJASTR, 3, 6, 2013, 787-797.
- Abo-Dief, H; Al-Ghamdi, S; Al-Zahrani, E; and Mohamed, A; The AES-ATEMA’2014 Seventeen Int. Conf. Montreal, CANADA, 2014, 201-211.
- Abualnaja, K; and Abo-Dief, H; The IJCM Engineering Vol: 4, No: 6, 2017.
- Vera, R; Schrebler, R; Cury, P; Del Rı´o, ; Romero, R; J Appl Electrochem 2007, 37, 519–525
CrossRef - Abo-Dief, H; Emam, A; Abualnaja, K; And Mohamed, A; Under Publication, Oriental Journal Of Chemistry, 2018, Vol. 34, No.(2).
- Sherif, E; Es-saheb, M; El-Zatahry, A; kenawyand, E; and Alkaraki; Int. J. Electrochem. Sci., 7, 2012, 6154 – 6167.
- Pujari, S; Ramakrishna, A; and Kumar, M; IJCT, Special Issue-2, 2014, 120-126.
- Alaoui, K; El Kacimi, Y; Galai, M; Touir, ; Dahmani, R; Harfi, A; and Ebn Touhami, M; J. Mater. Environ. Sci. 7, 7, 2016, 2389-2403.
- Gehlot, P, Daga, K; and Mehta, R; Int. J. of Chemistry, V. 3, 3; 2011, 56-61.
CrossRef - Senthilvadivu, B; Aswini, V; and Kumar, K; IJSR, V. 6, 7, 2017, 1762-1768.
CrossRef - Sangeetha, M; Rajendran, S; Sathiyabama, J; and Prabhaka, P; J. Nat. Prod. Plant Resour., 2012, 2 (5), 601-610.
- Palanisamy, K; Devabharathi, V; and Sundaram, N; IJCTR,7, 2015, 1661-1664.

This work is licensed under a Creative Commons Attribution 4.0 International License.









