Thermodynamics of Ion Exchange Reaction in Predicting the Ionic Selectivity Behavior of UV Radiation Degraded Nuclear Grade and Non-Nuclear Grade Resins
Department of Chemistry, Bhavan’s College, Munshi Nagar, Andheri (West), Mumbai, 400058, Maharashtra, India.
Corresponding Author E-mail: ashokpatange78@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404044
Article Received on : 09-05-2018
Article Accepted on : 21-06-2018
Article Published : 13 Jul 2018
The study represents the evaluation of performance of structurally related resins DuoliteARA 9366 and Duolite A-378 exposed to UV radiations of wavelength λ=254nm and λ = 384nm for 24 h in a UV Chamber and equilibrated separately with iodide ion solutions of different concentrations between temperature of 30.0oC - 45.0oC for 5 h .the equilibrium constants (K) values for Cl-/I- reactions with temperature for DuoliteARA-9366 were decreases from 21.57 x 10-2 to 15.57 x 10-2 for resin degraded at UV radiation of 384nm was lower than the decrease in K values from 26.22 x 10-2 to 19.92 x10-2 observed for the resin degraded at UV radiation of wavelength λ=254nm. Similar results were obtained for DuoliteA378 .The UV degradation of the resin surfaces were recognized by SEM and IR. The high K and low enthalpy values obtained for DuoliteARA-9366 shows that DuoliteARA-9366 was more selective towards iodide ions in solution than DuoliteA-378.
KEYWORDS:Duolite ARA-9366; Duolite A-378 UV Radiation Degradation; Fourier Transform Infrared Spectroscopy (FTIR) and (SEM) Nuclear Grade Resins; Scanning Electron Microscopy
Download this article as:| Copy the following to cite this article: Patange A. N. Thermodynamics of Ion Exchange Reaction in Predicting the Ionic Selectivity Behavior of UV Radiation Degraded Nuclear Grade and Non-Nuclear Grade Resins. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Patange A. N. Thermodynamics of Ion Exchange Reaction in Predicting the Ionic Selectivity Behavior of UV Radiation Degraded Nuclear Grade and Non-Nuclear Grade Resins. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47313 |
Introduction
Ion exchange is a process in which the mobile ions from external solutions were exchange with the ions that are bound to different functional groups in the resins if the resin containing positively charged functional groups then the exchange involves negative ions in the external solution and if the resin contains negatively charged functional group then it will remove or exchange positively charged ions from external solutions. This principle of ion exchange were used in different industries1 and factories like chemical, nuclear, food,pharmaceutical and color, drugs and dyes industries in order to remove the unwanted hard cations and hard anions in the process of manufacturing ,synthesis and purification. Ion exchangers were also used as phase transfer catalysis2 in various potential pharmaceutical as important intermediate for drug and dyes synthesis. In recent years most of research work were carried out on characterization studies of various nuclear and industrial grade organic as well as inorganic ion exchanger under various stringent chemical, thermal,γ-rays radiation degradation3 condition so as to improve the performance, efficiency and selectivity behavior towards variety of cations and anions in the industrial waste effluents, purification and manufacturing process4 and the removal of radio nuclides from nuclear industries.in order to remove or separate radio nuclides and other ions from industrial waste it was very difficult to choose the suitable ion exchange resins for the separations of these ions so that there was need of some thermodynamic data or techniques which will provides the useful information about the various nuclear and industrial grade resins and helps to select which of the ion exchange resins were suitable for removal or separations of various cations and anions from industrial waste and nuclear waste. Taking in to the considerations and needs of industries attempts were made in the present research study to evaluate the performance6 of two closely related resins viz Duolite ARA9366 and Duolite A-378 under stringent degradation conditions like UV radiation of different wavelength in a UV chamber7 for 24h separately for the resins and thermodynamic ion exchange equilibrium studies were carried out in predicting the selectivity behavior of these resins in chloride form towards the iodide ions of different ionic concentrations under the temperature range of 30.0 – 45.0oC for 5 h. the thermodynamic data obtained from the study will helpful in predicting the selectivity behavior of the resins. The data obtained from the study will also helpful for the selection of suitable ion exchange resin under the stringent degradation like UV radiations. The ion exchange equilibrium studies indicates that both resins shows strong absorption towards the longer UV wavelength at λ=384nm duo to the presence of conjugations and presence of quaternary and secondary amino groups .for irradiation of the resins in UV radiation for longer period of time in UV chamber blocks the particular sites of the resin by reducing the ion exchangeable groups which will responsible for the decrease in the equilibrium constant values and the enthalpy values of for Cl–/I– ion exchange reactions and consequently8 affects on the performance of the resins viz Duolite ARA9366 and Duolite A-378 which will also supported by the SEM micrographs and IR spectra of the degraded resin surfaces.
Materials and methods
Materials
The anion exchange resin DuoliteARA9366 and DuoliteA-378 in hydroxide9 form was supplied by the Auchtel chemicals limited, Mumbai were strongly basic nuclear grade and weakly basic non-nuclear grade anion exchange resin. The resin granules of mesh size 30-40 were used for the research work. Both the resins converted into chloride ion form for the experimental study by treating the respective resin sample with 20% KCL solution for 24h in a 50cm long and 2.5cm in diameter glass column at the rate of 1ml for 30seconds.after conditioning of the resin sample in chloride form were purified with help of distill water and ethanol solvent and air dried and stored in glass bottle.
UV radiation degradation of resins
In order to predict the performance and selectivity nature of the resins about 50g of both resin10 sample in chloride form placed separately in an a UV chambers and irradiate them for 24 hours continuously under UV radiation of wavelength λ=254nm and λ=384nm.after 24 hours both resin sample were drawn from the UV chambers11 and washed with distilled water and then with ethyl alcohol and air dried and stored in glass bottle and used for further study.
Ion Exchange Equilibrium Study
For equilibration study about 1g of UV radiation degraded resins viz DuoliteARA9366 and DuoliteA-378 in chloride form were equilibrated12 separately with iodide ion solution of different ionic concentration from 0.01M, 0.02M, 0.05M, 0.08M and 0.10M on well-equipped magnetic stirrer with hot plate and automatic temperature control at the different temperatures from 30.0oC to 45.0oC for 5h as explained12 After 5 hours, the quantity of iodide ions transfers with the resin surface can be determined potentiometricallyby using standard 0.01M AgNO3 using saturated calomel electrode as reference and silver electrode as indicator.
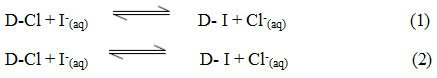
Where D=Duolite ARA-9366 = Duolite A-378
The equilibrium constants K for the reaction (I) and (II) at different temperature from 30.0oC to 45.0oC
Were calculated.
Results and Discussion
The equilibrium constants (K)16 for reactions I and II were calculated by the equation.
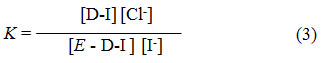
Here, D = Duolite ARA-9366 = Duolite A-378 Surface; E = ion exchange capacity of the resin .Thus by knowing13 different ionic concentrations or amount of I– ions in solution transfers on the respective resin surface at a given temperature, K- values and mean K-values for this batch of experiment was determined. Similar K values were obtained for the Cl–/I– exchange reactions at different temperatures. the standard ΔHo, ΔGo and ΔSo in kJ.mol-1 of Cl–/I– ion exchange reactions for UV radiation degraded resin sample were determined from the slope obtained by plotting a graph of log K against 1/T (0K) 14 (figure 1-4). The thermodynamic parameters calculated for Cl–/I– reactions of fresh resins viz Duolite ARA9366 and Duolite A-378 and resins exposed to Degradation at different UV wavelength were represented in Tables 1 to 4.
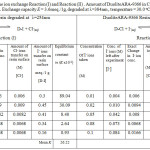 |
Table 1: Equilibrium constant for the ion exchange Reaction (I) and Reaction (II). |
Amount of Duolite ARA-9366 in Cl– form = 1g ,Exchange capacity E = 3.48meq./1g, degraded at λ=254nm. Exchange capacity E = 3.6meq./1g, degraded at λ=384nm , temperature = 30.0oC
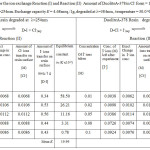 |
Table 2: Equilibrium constant for the ion exchange Reaction (I) and Reaction (II). |
Amount of DuoliteA-378 in Cl– form = 1g , Exchange capacity E = 4.62meq./1g, degraded at λ=254nm. Exchange capacity E = 4.68meq./1g, degraded at λ=384nm , temperature = 30.0oC
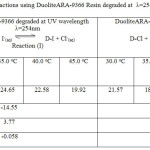 |
Table 3: Thermodynamics Cl–/I– reactions using DuoliteARA-9366 Resin degraded at λ=254nm and λ=384nm |
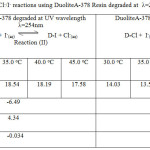 |
Table 4: Thermodynamics Cl–/I– reactions using DuoliteA-378 Resin degraded at λ=254nm and λ=384nm Click here to View table |
On observing the equilibrium constant (K)-values from Table 3-4 for Cl–/I– ion exchange reactions, the K-values for both UV degraded resins viz Duolite ARA9366 and Duolite A378 were found to be decreases with rise in temperature from 30.0oC to 45.0oC shows that Cl–/I– ion exchange reactions are exothermic in nature. The High K-values and low enthalpy values obtained for Duolite ARA9366 as compare to the K-values for Duolite A-378 resin indicates that the Resin Duolite ARA9366 was very selective or having greater affinity towards the iodide ions in solution as compares to Duolite A-378.the equilibrium constant (K-values) and enthalpy values obtained for both resins viz Duolite ARA9366 and Duolite A378 degraded at =384nm will be lower than that the K-values and enthalpy values obtained for resins Duolite ARA9366 and Duolite A-378 exposed to UV light at =254nm this will shows that both resins strongly absorbs UV radiation of 384nm which will results in decreasing the ion exchange functional groups in the polymer chain of the resin and lowers the rate of ion exchange reactions at the resin surface and decreasing the equilibrium constant value for ion exchange process. This also seen in the IR spectra and SEM images of the degraded resin surfaces (figure 5-16).
Graphs
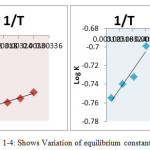 |
Figure 1-4: Shows Variation of equilibrium constant for Cl–/I. with temperature Duolite ARA-9366 & Duolite A-378 degraded at UV wavelength λ=254nm and λ=384nm |
IR Analysis of DuoliteARA-9366 and DuoliteA-378Resin degraded at λ=254nm and λ=384nm
IR spectra of fresh as well as degraded samples of Duolite ARA-9366 and Duolite A378 resins15 were scan on Perkin Elmer 1750 FTIR spectrophotometer. Most of characteristic functional group of both fresh as well as UV degraded resins was found to be appears at the theoretical values but some considerable changes in some characteristic IR band were found to be broad in degraded resin sample duo to the strong absorption of UV wavelength at λ=384nm and crack the resin surface and lowers rate of ion exchange process which are also seen in SEM of both UV degraded resin sample.
 |
Figure 5-7: FTIR Spectrum of fresh resin, Duolite ARA-9366 Resin degraded at λ=254nm and λ=384nm. |
 |
Figure 8-10: FTIR Spectrum of fresh resin, DuoliteA378 Resin degraded at λ=254nm and λ=384nm. |
 |
Figure 11-13: FTIR Spectrum of fresh resin, DuoliteARA9366 Resin degraded at λ=254nm and λ=384nm. |
 |
Figure 14-16: FTIR Spectrum of fresh resin DuoliteA-378 Resin degraded at λ=254nm and λ=384nm. |
SEM images of fresh and degraded resin surfaces were made exposed electronically in a vacuum on thin carbon layer for 60 seconds at 30 W and pictures were captured with help of JSM-6380LA model16. On comparing the SEM micrographs of fresh as well as UV irradiated resin surfaces, it will found that both resin were strongly absorbs UV radiation of wavelength 384nm as comparing to 254nm and due to this strong absorption 384nm UV light surfaces17-18 of resin cracks and appears to more rough and enough to block the ion exchange site of the resin and consequently lowers down the ion exchange process as compare to fresh resin surfaces.
Conclusion
The thermodynamic data obtained from the research study will be helpful in selecting the suitable ion exchange material for the removal of various cations and anions in the industrial waste, nuclear waste and removal of unwanted interfering ions in organic synthesis process. The results obtained from the work will also helpful for qualitatively assessing the efficiency, economy and performance of resins under the vigorous condition like chemical, thermal as well as under electromagnetic radiations.
References
- Wiley, J. ; and sons. Inc. J.Appl polym Sci. 1997, 64; 1161-1167.
CrossRef - Baumann, E. ; J. Chem. and Eng. Data, 1960, 5, 376.
CrossRef - Singare P.U; Patange,A.N.;ILCPA, 2014,11, 67.
CrossRef - Singare P.U; Patange,A.N.;, ILCPA, 2014,11, 44.
CrossRef - Singare P.U; Patange,A.N.; ILCPA, 2014, 6, 8.
CrossRef - Singare P.U; Patange,A.N.; ILCPA, 2014,6, 1.
CrossRef - Patange,A.N.; Orient.J.Chem. 2017. 33(1),430-438.
CrossRef - Bhargava, A.; Janardanan,C.; Indian J.Chem.,1997,36A, 624 .
- Lokhande,R.S.; Singare,P.U.; Patil ,A.; Russ. J. Phys. Chem. A,2007,81,2059.
CrossRef - Patange,A.N.;GJESR, 2017, 4(8), 57-71.
- Lokhande,R.S.; Singare, P.U; Radiochim. Acta, 2007,95,173.
- Lokhande,R.S.;Singare,P.U.; J .Porous Mater, 2008,15, 253 .
CrossRef - McNeill,I.C.;Mohammed,M.H.; Polym. Degrad. Stab. , 1995,48,175 .
CrossRef - Patange,A.N ;Singare P.U; Res.J.Pharm.,Biol. Chem.Sci., 2017, 8(2),1866-1879.
- Jiang,D.D.; Yao,Q.;McKinney,M.A.;Wilkie,C.A.; Polym. Degrad. Stab.,1999,63, 423 .
CrossRef - Decker , C.; Zahouily,K.; Polym. Degrad. Stab. , 1999, 64,293 .
CrossRef - Santhiya, D.; Subramanian,S.; Natarajan ,K.A.; Malghan,S.G.; J. Colloid. Int. Sci., 1999,216,143.
- Kaczmarek,H.; Felczak,A.; Szalla,A.; Polym. Degrad. Stab. ,2008, 93,1259.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










