The Study of Physical and Chemical Properties of the Water-Soluble Polymer Reagents and Their Application as an Ointment
Nagima Dzhakipbekova , Saule Sakibayeva, Erzhan Dzhakipbekov, Didar Ahmet, Saparkul Rzabay, Aziza Issa, Aigul Kydyralyeva and Eltay Amantayev
, Saule Sakibayeva, Erzhan Dzhakipbekov, Didar Ahmet, Saparkul Rzabay, Aziza Issa, Aigul Kydyralyeva and Eltay Amantayev
M. Auezov South Kazakhstan state university, Shymkent, Republic of Kazakhstan.
Corresponding Author E-mail: isa.aziza@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/3404010
Article Received on : 20-06-2018
Article Accepted on : 03-07-2018
Article Published : 20 Aug 2018
The creation of new polymer reagents from their synthesis in the laboratory to their industrial production as an ointment is relatively long and expensive process. Therefore, the most promising and justified way is to expand the range of polymer reagents by modifying the already known base samples. We studied MPAA(HP)- hydrolyzed polacrylamide modified with hydrogen peroxide,, MPAA (MEA) - hydrolyzed polycrylamide modified with monoethanolamine, MPAA (TEA) - hydrolyzed polycrylamide modified with three ethanolamine polymers. A more detailed and in-depth study of them will expand the scope of application of low-toxic, affordable reagents that are of equal efficacy to the already existing ones and their application as an ointment. Polymer MPAA-HP polymacrylamide hydrolyzed NaOH with the addition of the H2O2 modifier in the ratio PAA: NaOH: H2O2 = 1: 0.4: 0.2 the hydrolysis reaction is carried out for 1.5-2 hours at temperature of 90-950C. MPAA-MEA polymer polymaracrylamide is hydrolysed with alkali with the addition of monoethanolamine modifier in the ratio of PAA: NaOH: MEA = 1: 0.4: 0.1 the hydrolysis reactions is carried out for 1.5-2 hours at temperature of 90-95°C.
KEYWORDS:Density; Ethanolamine; Monoethanolamine; MPAA (HP) - Hydrolyzed Polacrylamide Modified With Hydrogen Peroxide; MPAA (MEA) - Hydrolyzed Polycrylamide Modified With Monoethanoamine; MPAA (TEA) - Hydrolyzed Polycrylamide Modified With Three Ethanolamine; Polyelectrolyte; Viscosity
Download this article as:| Copy the following to cite this article: Dzhakipbekova N, Sakibayeva S, Dzhakipbekov E, Ahmet D, Rzabay S, Issa A, Kydyralyeva A, Amantayev E. The Study of Physical and Chemical Properties of the Water-Soluble Polymer Reagents and Their Application as an Ointment. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Dzhakipbekova N, Sakibayeva S, Dzhakipbekov E, Ahmet D, Rzabay S, Issa A, Kydyralyeva A, Amantayev E. The Study of Physical and Chemical Properties of the Water-Soluble Polymer Reagents and Their Application as an Ointment. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48673 |
Introduction
The creation of new polymer reagents from their synthesis in the laboratory to their industrial production as an ointment is relatively long and expensive process.
Therefore, the most promising and justified way is to expand the range of polymer reagents by modifying the already known base samples. We studied MPAA(HP)- hydrolyzed polacrylamide modified with hydrogen peroxide,, MPAA (MEA) – hydrolyzed polycrylamide modified with monoethanolamine, MPAA (TEA) – hydrolyzed polycrylamide modified with three ethanolamine polymers. A more detailed and in-depth study of them will expand the scope of application of low-toxic, affordable reagents that are of equal efficacy to the already existing ones and their application as an ointment.
Objects of research
Characteristics of the studied MPAA(HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA(MEA) – hydrolyzed polycrylamide modified with monoethanoamine Copolymers.
Polymer MPAA-HP polymacrylamide hydrolyzed NaOH with the addition of the H2O2 modifier in the ratio PAA: NaOH: H2O2 = 1: 0.4: 0.2 the hydrolysis reaction is carried out for 1.5-2 hours at temperature of 90-95°C.
MPAA-MEA polymer polymaracrylamide is hydrolysed with alkali with the addition of monoethanolamine modifier in the ratio of PAA: NaOH: MEA = 1: 0.4: 0.1 the hydrolysis reactions is carried out for 1.5-2 hours at temperature of 90-95°C.
Copolymers of acrylic acid, which are well mixed with water forming a viscous fluid mass, were synthesized on the basis of the Department of physical and colloid chemistry in M. Auezov SKSU. Their properties and consistency are of interest for the possibility of their use as auxiliary substances in pharmaceutical technology in the preparation of external medical forms.
The preparations have undergone poison control. On the basis of toxicological studies and analysis of the data obtained, it was concluded that polymers have a slight functional cumulation. They do not show skin-blistering and allergic effects.
MPAA(HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA(MEA) – hydrolyzed polycrylamide modified with monoethanoamine. Viscosity (ŋrel) of 0.2% solution is 4.12 – 5.64. Over time, the viscosity increases. Within 24 hours after synthesis, it is 5.64; after 30 days ŋ=4,12, that is 36 times increase.1 You can use this property when you obtain films.
Granulated polyacrylamide (PAA)
GPAA is granules of irregular shape, yellowish or slightly brown. The mass fraction of the polymer in the product is 50-56%, moisture is 10-14%, ammonium sulphate is 30-40%. The pH of 0.1% aqueous solution is 7-8. The kinematic viscosity of 0.1% aqueous solution is not less than (1.7 – 2) 10 -6.2
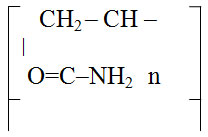
Characteristics of Medical Preparations Used in the Work
Antibacterial Preparation
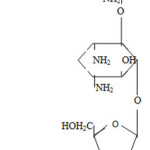
Neomycin sulfate
Neomycin is a complex of antibiotics (neomycin A, B, C) formed during the life of the radiant fungus Streptomycess fradiae: in the form of sulfates they are part of the preparation neomycin sulfate.4
The main component of the neomycin complex (more than 90%) is neomycin B, which has a small antimicrobial activity. The antibiotic is active against pathogenic staphylococci, corynebacteria, listeria, anthrax and most gram-negative bacteria (Escherichia coli, Shigella, Salmonella, etc.).
 |
Scheme 1 |
Neomycin sulfate is a white and yellowish-white fine crystalline powder almost odorless, easily soluble in water, insoluble in alcohol and insoluble in other organic solvents. It is hygroscopic. Aqueous solution at pH 2.0-9.0 is stable (at pH 8,0 stable for a year).
The characteristic physical constant is the specific rotation from + 50 to + 580 (5% aqueous solution of the preparation).5-7
To test the authenticity of neomycin sulfate color reaction with alcoholic solution of orcein and concentrated hydrochloric acid in the presence of iron (III) chloride is used. The drug forms green substance by heating the reaction mixture.8
Biological activity is determined by diffusion in agar with test microbes.6 Cereurvar bacillus mycoides is used as the microbal test.4
Levomycetin – Laevomycetinum
A synthetic substance identical to the natural antibiotic chloramphenicol, which is a product of the vital activity of the micro-organism Streptomyces venezuelae.9 In industry, antibiotic production is carried out mainly by chemical synthesis.10
The chemical structure of the drug is a D-(-)-three-1-para-Nitrophenyl-2-dichloracetylamin-propanediol-1,3.
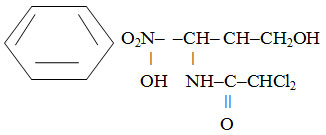
It is white or white with a weak yellowish-greenish tinge crystalline powder with bitter taste. It is slightly soluble in water, easily soluble in alcohol.8
Laevomycetini succinatis solubile is a soluble form of Levomycetin.
Chemically, it is D – (-) – three-1-para-Nitrophenyl-2-dichloroacetylamino-1,3-propanediol – 3 sodium succinate:
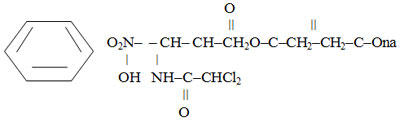
It is a dry porous mass of white or white with a yellowish tint with a weak specific smell and bitter taste. It is very easily soluble in water, slightly soluble in alcohol. It is hygroscopic. pH of 30% aqueous solution is 6.4-7.5.10
The drugs have a wide spectrum of antimicrobial activity, they are active against many Gram-positive and Gram-negative bacteria, rickettsia, spirochaete, chlamydia.9
To test the authenticity of both drugs, hydrolytic cleavage reactions are used in alkaline and acidic media, followed by identification of the products formed.
For qualitative and quantitative determination of levomycetin and ascomycetin succinate, spectrophotometry in the UV region is used at a wavelength of 278 nm and 275 nm, respectively.
The quantitative determination of levomycetin is performed by a nitrite-metric method after preliminary reduction in acid medium by zinc dust according to the USSR pharmaceutical regulations.10
Local Anesthetic Preparation
Novocainum
β-diethylaminoethyl ester of paraminobenzoic acid hydrochloride:
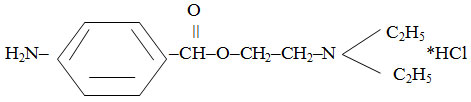
They are colorless crystals or white crystalline powder without odor, with bitter taste. It is easily soluble in water (1: 1), easily soluble in alcohol (1: 8). Melting point: 154-1560С.
The authenticity is established by the reactions of azo dye formation with β-naphthol, also with some precipitation (total alkaloid) reagents (picric, phosphoric tungsten, phosphomolybdenum acids).
Quantitative determination is carried out by titrometric method according to USSR pharmaceutical regulations.
The preparation represents hydrochloride and can be quantified by the method of neutralization according to the bound hydrochloric acid.10
Research methods
When developing the ointment base optimal composition, 1.0 g of MPAA polymer was dissolved in different volumes of distilled water: 1 ml, 2 ml, 3 ml, 4 ml, 5 ml and 6 ml.
It was found out that to obtain an ointment base with the necessary consistency, the optimal ratio of MPAA polymer and distilled water was 1: 5.
The pre-ground and screened MPAA polymer powder was weighed on a BP-1 scale in an amount of 1.0 g, transferred into a mortar and triturated with 5 ml of distilled water, added in parts. As a result of a ten-minute stirring, a thick, sticky, gel-like, transparent mass without odor, yellowish-cream color was obtained. The base is easily applied to the skin, when drying it forms a thin protective film on it, easily removable with a cotton swab dipped in water. The base does not spoil underwear and clothes, does not have irritating effect and does not tighten the skin when it dries.
Thus, the technology of this synthetic hydrophilic base preparation is extremely simple, does not require special methods of preparation and compliance with a lot of conditions. It is convenient and quick to use.
Methods of Polymer Research
Conductivity
pH of MPAA (HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA (MEA) – hydrolyzed polycrylamide modified with monoethanoamine, MPAA (TEA) – hydrolyzed polycrylamide modified with three ethanolamine polymers solutions was measured on a potentiometer pH-340 with an accuracy of +/-0.05 in a thermostatically controlled cell in which the temperature was maintained with an accuracy of 25+/-0.01°C. Further, the electrical conductivity was determined by the formula (x):
![]()
where α is the cell constant, it was determined by the formula:
α=0.000147 m-1cm-1 at T=250°C
Electrical conductivity of GRP MPAA-HP, MPAA-MEA, MPAA-TEA solutions is studied3 on the device assembled according to the bridge circuit with the frequency of 1000Hz; voltage was produced by the ZG-6m sound generator. The output voltage is 6V. The electrical cell has the form of a cylindrical vessel with a capacity of 25 ml; electrodes are made of blackened platinum. The arrangement of electrodes is vertical. The thermostating of the cell is carried out in a water thermostat. The cell constant (K) at 298 K is 0.37.
Specific conductivity (χ) is calculated according to the formula:
χ = K/R х (Ом-1×sm-1),
where: R- is resistance (Ohm-1) of the studied solution;
K- is cell constant.
Potentiometric titration is carried out on a laboratory pH meter – millivoltmeter LPM-60 m with a sensor DL-01 at 298±0,1 K in a nitrogen current. The meter is a glass electrode ESL IIG-0.4, and comparison electrode is calomel electrode.1-3
Physical, chemical and microbiological research methods according to the following techniques were used.
Method 1. Determination of Antibiotics Antimicrobial Activity by Microbiological Method Of Diffusion In Agar
To establish compatibility of the copolymers with the antibiotics levomycetin, levomycetin sodium succinate and neomycin sulphate it is necessary to reveal their antimicrobial activity, which is determined by the method of diffusion in agar. The methodology of this research is described in detail in USSR pharmaceutical regulations.
Due to the fact that the method does not take into account some features of our experiment (for example, the determination of antimicrobial activity in films), we have modified the method in order to be able to use it in our work.
The diffusion in agar method is based on comparing the degree of the test-microbe growth zone inhibition by certain concentrations of the antibiotic in the test material with the inhibition of its growth zone by the known concentrations of the standard antibiotic. Suppression of the growth of the test-microbe is carried out by diffusion of the antibiotic from the studied material into a dense nutrient medium.5
To obtain reproducible results, strict standardization of experiments is necessary. The rate of solutions diffusion in agar depends on the chemical nature of the antibiotic, the composition, pH of the agar medium, the buffer in which the working solutions of the standard and the test material are prepared, the temperature and incubation time. Therefore, when determining the concentration of antibiotics and maintenance of their antibacterial activity in test samples we should select the conditions for culturing the test culture, the optimal composition and pH of medium, buffer solutions, ensuring the maximum diffusion of the solutions of antibiotic into the medium and the sharpness of the zones outlines. The determination is carried out according to the scheme common to all antibiotics, which consists of several stages.4
Step 1. Preparation of nutrient medium
Base Hottinger broth was diluted 5 times in distilled water. 0.5% sodium chloride solution, 0.1% solution of single potassium phosphate was added. pH was set to 7.4-7.6 and the solution was boiled for 20 minutes.
The broth prepared in this way was filtered through a cotton-gauze filter. Then agar-agar “Tafuinsky” (15 g of agar per 1,000 ml of broth). The broth is poured into bottles and sterilized under pressure at a temperature of 1200С for 30 minutes.7,8
Step 2. Preparation of dishes with media
Medium in a flask is melted in a water bath (removing the paper caps beforehand). After the medium has become liquid, the flask is taken into the right hand, the cork is removed with the left hand over the fire and is held between the little finger and palm, and the edges of the flask are burned.
The open dish with the medium is kept in an inclined position to avoid the ingress of microorganisms from the air. At the same time, the left hand is raising the lid on a sterile Petri dish on the one side and pouring 20 ml of nutrient medium into it, gently rocking the dish so that the medium is distributed evenly over its entire surface. The thickness of the layer is 5 mm. During gelling of the agar, a large amount of condensation water forms and settles on the inner side of the dish lid, moistening the surface of the medium. Then, after gelation of the medium, the dishes are dried for 30 minutes with the slightly open lid.7,8
Step 3. The preparation of inoculum and contamination of the dishes
Determination of the antibiotics activity in the studied basics is carried out with pure cultures of Escherichia coli (ATSS 25922), Straphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853). The method of mechanical separation of microbes by Drigalsky18 was used to separate the pure culture of microorganisms from the pathological material, which makes it possible to obtain isolated colonies on the surface of the nutrient medium.
To do this, the pathological material is distributed by empty parallel strokes throughout the dish in one direction. Then, rotate the cup by 1800, and using the same loop (without burning it) and not picking up new material, conduct the finishing touches in the direction perpendicular to the first strokes. As a result, a dense grid is drawn on the surface of the nutrient medium. With this method of sowing, the pathological material contained on the loop is consumed gradually, and along the lines of the grid applied at the end of sowing. Then isolated colonies of microbes begin to grow.9
After isolation of pure culture 5-10 homogeneous colonies are suspended in 2 ml of liquid medium (Hotinger broth).
The density of the suspension must comply with the standard turbidity № 10 (1 billion of microbial bodies in 1 ml). The bacterial mixture is poured onto the surface of the medium and evenly distributed by dangling the dish. Excess fluid is removed with a pipette. Dishes are dried at room temperature for 30 minutes.10
Step 4. Preparation of working solutions of antibiotic standard test material
For the preparation of standard antibiotic solutions random, but the exact sample was dissolved in an appropriate solvent. For levomycetin sodium succinate and gentamicin sulfate phosphate buffer (pH 7.8-8.0) of the following composition was used as a solvent: potassium monophosphate – 0,68 g, disubstituted sodium phosphate – 10,99 g, distilled water up to 1000 ml. 1 mg of the sample or 1000 units was dissolved in 1 ml of phosphate buffer (basic solution). Further dilutions were prepared by further dilution of the main solutions to the desired concentrations.
Due to the fact that the linear correlation between the concentration and the size of the growth delay zones of the test microbe is detected only in a certain concentration range, the concentrations of solutions of the standard antibiotics should be accurately selected. For the standard of each antibiotic, the concentration of the solution is determined, which would provide the formation of optimal zones of growth delay of the test culture (control concentration). Such concentrations for levomycetin sodium succinate sulfate are 30 µg/ml and 4.0 µg / ml, respectively.5
Step 5. Preparation of holes in the nutrient medium
Round holes of the regular shape with a diameter of 8 mm and a depth of 4 mm are made with a cork drill at a distance of 2.5 cm from the center of the dish and at an equal distance from each other in the frozen agar. The prepared solutions of the antibiotic standard and the test sample are applied to the holes and kept for 3 hours at room temperature. Then the cups are thermostated for 18 hours at a temperature of 37°C upside down so that condensation water does not get on the sowing surface, which could distort the results of the experiment.
Step 6. Records of the results
Measurement of growth inhibition of the test microbe zones, formed by the samples and standard solutions was carried out and compared. The results of the experiment are assessed by the change of antibacterial activity of antibiotics in the ointment samples.
The application of MPA-PV, MPA-MEA polymers in combination with antibacterial drugs was studied.
Results and Discussion
The property of MPAA (HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA (MEA) – hydrolyzed polycrylamide modified with monoethanoamine, MPAA (TEA) – hydrolyzed polycrylamide modified with three ethanolamine polymers to dissociate in aqueous solution allows to study the electrical conductivity of aqueous solutions of polymers and to research the ionizability of macromolecules in solution. It is known that electrical conductivity depends on the concentration of polymers. With the increasing concentration of the studied samples of GRP, the specific conductivity increases, and then this dependence is exponential. Thus χ depends on a ratio of polymers functional groups.
In such systems, it is seen that the distribution of mobile ions between the areas occupied by poly ions and in the surrounding space is not regulated by Donnan balance. The activity coefficients of ions must be compressed at the expense of the existing electrostatic forces, which lead to a decrease in the potential and, accordingly, in their electrical conductivity. In addition, the possible formation of ion pairs, associates, chelates formed due to the hydrogen bonds can take place.
Figure 1 shows that the initial incident portion of the curve corresponds to the region of complete ionization of functional groups and thermodynamic stability of the system.
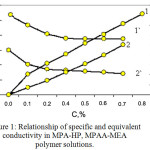 |
Figure 1: Relationship of specific and equivalent conductivity in MPA-HP, MPAA-MEA polymer solutions. |
Specific (1, 2) and equivalent (1`,2`) conductivity of solutions: 1-MPAA-HP; 2-MPAA-MEA
Straight section parallel to the absciss axis corresponds to the area of structured solutions in which macromolecules are associated. The fracture on the curve can be considered as the beginning of conformational transformation of macromolecular tangles in MPAA-HP, MPAA-MEA polymer solutions with the possible formation of supramolecular structures in concentrated polymer solutions.
In all MPAA-HP GRP samples, the pH-q correlation has S-shaped form with inflection points in the acidic (3.5-4.0) and alkaline9-10 areas (figure 2). In an acidic medium (pH=3.5-4.0), COOH groups slightly dissociate and are in a tangled state; when alkali is added, the neutralization of hydrogen ions mainly occurs at higher pH. The neutralization reaction is accompanied by the appearance of charges on the macromolecule, which affects the titration curve and the chain shape.
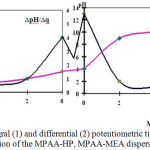 |
Figure 2: Integral (1) and differential (2) potentiometric titration curves of 0.1% solution of the MPAA-HP, MPAA-MEA dispersions mixture. |
Curves with inflection points in the acidic and alkaline regions indicate the presence of both acidic and basic functional groups. Fully titrated acidic and basic groups correspond to extreme points on the differential curves of potentiometric titration.
For MPAA-HP, MPAA-MEA amphoteric polymers, the titration curve looks more complicated than for polyacids and polybases.
Carboxyl groups of MPAA-HP, MPAA-MEA are titrated in the pH range=4-9. At pH below four, hydrochloric acid joins the amide and imide groups to form the salt of hydrochloric acid polyamide, meanwhile pH changes to three. Earlier it was established that for a polypeptide chain electrolytic interaction of closely spaced groups differs for spiral and tangle-shaped conformations; in the transition spiral-tangle region, the degree of ionization of the macromolecule increases sharply when the pH of the solution changes.
The electrical conductivity of solutions MPAA-HP, MPAA-MEA (figure 3) is studied.
Increase in the concentration of MPAA-HP, MPAA – MEA solutions leads to an increase in specific conductivity up to a certain concentration. Then this correlation becomes exponential. This can be explained by either the fact that not all ionogenic groups of the polymer are ionized, or that some factors impede electricity transfer.
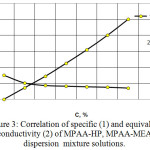 |
Figure 3: Correlation of specific (1) and equivalent conductivity (2) of MPAA-HP, MPAA-MEA dispersion mixture solutions. |
When developing the ointment base optimal composition, 1.0 g of MPAA polymer was dissolved in different volumes of distilled water: 1 ml, 2 ml, 3 ml, 4 ml, 5 ml and 6 ml.
It was found out that to obtain an ointment base with the necessary consistency, the optimal ratio of MPAA polymer and distilled water was 1: 5.
The pre-ground and screened MPAA polymer powder was weighed on a BP-1 scale in an amount of 1.0 g, transferred into a mortar and triturated with 5 ml of distilled water, added in parts. As a result of a ten-minute stirring, a thick, sticky, gel-like, transparent mass without odor, yellowish-cream color was obtained. The base is easily applied to the skin, when drying it forms a thin protective film on it, easily removable with a cotton swab dipped in water. The base does not spoil underwear and clothes, does not have irritating effect and does not tighten the skin when it dries.
Thus, the technology of this synthetic hydrophilic base preparation is extremely simple, does not require special methods of preparation and compliance with a lot of conditions. It is convenient and quick to use.
Conclusion
The composition and conditions for obtaining MPAA (HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA (MEA) – hydrolyzed polycrylamide modified with monoethanoamine polymers were determined.
Polymer MPAA-HP polymacrylamide hydrolyzed NaOH with the addition of the H2O2 modifier in the ratio PAA: NaOH: H2O2 = 1: 0.4: 0.2 the hydrolysis reaction is carried out for 1.5-2 hours at temperature of 90-95°C.
MPAA-MEA polymer polymaracrylamide is hydrolysed with alkali with the addition of monoethanolamine modifier in the ratio of PAA: NaOH: MEA = 1: 0.4: 0.1 the hydrolysis reactions is carried out for 1.5-2 hours at temperature of 90-95°C.
The research of physical – and colloid-chemical properties of polymers revealed their polyelectrolyte nature; they relate to high-molecular surface-active substances (the electrical conductivity of aqueous solutions of polymers changes depending on the conformational transformations of the macromolecular coil of surfactants in Fig. 2 and Fig. 3.).
The studied polymers were researched in complex with the levomycetin, levomycetin sodium succinate and neomycin sulfate. The optimal composition of the ointment base is 1 g of polymer and 5 ml of distilled water.
The compatibility of MPAA (HP) – hydrolyzed polacrylamide modified with hydrogen peroxide, MPAA (MEA) – hydrolyzed polycrylamide modified with monoethanoamine, MPAA (TEA) – hydrolyzed polycrylamide modified with three ethanolamine polymers, with levomycetin, levomycetin sodium succinate and neomycin sulphate was shown and the antimicrobial activity of the antibiotics was revealed by the method of diffusion in agar.
References
- Tager A.A. Physics and chemistry of polymers. Moscow.: Goschimizdat, 1992, p.23-26
- Fuoss R, Strauss U.J. Polymer. Sci., 1998, 3.246
- Febbereitor K.J. Temperature dependence of surface tension // Coll.and Polym. Sci., 1978. V 256., №5, p.490-493.
- Belikov V.G. Pharmaceutical chemistry: A textbook for pharmaceutical institutes and pharmaceutical faculties of medical institutions. -M .: Higher School, 1985, 768 p
- Bilibin A.F. Clinical chemotherapy. – Moscow: Medicine, 1983, 511 p.
- Dmitrieva VS, Semenov S.М. Microbiological analysis of antibiotic activity. -M .: Medicine, 1984, 284 р.
- Kashkin P.N, Bezborodov A.M, Elinov N.T. Antibiotics. -M .: Medicine, 1970
- Pyatkin K.D. Microbiology with virology and immunology. – Moscow: Medicine, 1981, 347 p.
- Tentsova A.I, Azhgikhin I.O. Dosage forms and therapeutic activity of drugs. – Moscow: Medicine, 1974, 336p.
- Kondratieva T.S., Ivanova L.A, Zelikson Yu.I. Technology of medicinal forms. t.1. – M .: Medicine, 1991.

This work is licensed under a Creative Commons Attribution 4.0 International License.









