Synthesis and Anticancer Activity of Mixed Ligand, Cobalt (II), Nickel (II), Manganese (II) Complexes of Tertiary Diphosphines with Dithizone (H2dz)
Department of Chemistry, College of Education, University of Salahaddin- Erbil, Iraq.
Corresponding Author E-mail: hikmatmohamad@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/3404027
Article Received on : 26-05-2018
Article Accepted on : 04-07-2018
Article Published : 07 Aug 2018
The one-pot synthesis reaction of one mole MCl2.nH2O, where M= Co(II), Ni(II), Mn(II) ,with one mole of 1,5-diphenylthiocarbazone (dithizone;H2dz), of 1,1-bis(diphenyl phosphine)ferrocene (dppf) and 1,2-bis(diphenyl phosphine) ethane (dppe) gave colored complexes of; [Co(Hdz)(k2-dppf)]Cl, [Ni(Hdz)(k2-dppf)]Cl, [Ni(Hdz)(k2-dppe)]Cl and [Mn(Hdz)(k2-dppf)]Cl. The synthesized complexes have been identified by using 1HNMR, IR, UV-Vis spectroscopy, micro elemental analysis and molar conductance. All complexes were tested for their anticancer activities on Human breast cancer cell line CAL5. The results showed that [Ni(Hdz)(k2-dppe)]Cl and[Mn(Hdz)(k2-dppf)]Cl have a highest activities than cisplatin in compared to; [Co(Hdz)(k2-dppf)]Cl, [Ni(Hdz)(k2-dppf)]Cl.
KEYWORDS:Anticancer Activity; Cobalt; Dithizone; Manganese Complexes; Nickel; Tertiarydiphosphines
Download this article as:| Copy the following to cite this article: Mohamad H. A. Synthesis and Anticancer Activity of Mixed Ligand, Cobalt (II), Nickel (II), Manganese (II) Complexes of Tertiary Diphosphines with Dithizone (H2dz). Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Mohamad H. A. Synthesis and Anticancer Activity of Mixed Ligand, Cobalt (II), Nickel (II), Manganese (II) Complexes of Tertiary Diphosphines with Dithizone (H2dz). Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48073 |
Introduction
Extensive study of metal complexes as chemotherapeutic and drug agents is widely related to employment of cisplatin as an anticancer drug a above thirty years ago.1 But all the cisplatin is suffering from several affects like individual drug resistance. Above six years ago the status of cisplatin has been updated.2 The studies of N-heterocyclic Pt (II) complexes as reproducible of cisplatin have been showed that the planar substituted pyridine ligands in platinum complexes can be the mode of action of cisplatin.3,4
The medicinal uses and applicable of metals and metal coordination compounds are increasing drug and chemotherapeutic agents.5,6 The anticancer abilities of the 10 elements; As, Sb, Bi, Au; V, Fe, Rh, Ti, Ga, Pt have been reported.6
Dithizone has Thione-Thiol tautomer (Scheme 1) and dithizonate has isomeric form (Scheme 2). Dithizone has been reported as a good ligand because of its ability to form colored complexes with different metal ions. Dithizone is also used in medical applications for the treatment of prostate cancer and pain due to metastases.7,8
 |
Scheme 1: Thione –Thiol tautomers1, 5-diphenylthiocarbazone (dithizone;H2dz) |
 |
Scheme 2: Isomeric forms of dithizonate ion and its metal complexes. |
This work reports that the mixed ligand dithizone containing phenyl phosphine’s with Cobalt(II), Nickel(II), Manganese(II) formulas; [Co(H2dz)(k2-dppf)]Cl, [Ni(H2dz)(k2-dppf)]Cl, [Ni(H2dz)(k2-dppe)]Cl and[Mn(H2dz)(k2-dppf)]Cl. The anticancer evaluation activities were obtained on Human breast cancer cells, using cisplatin as a reference.
Experimental
Materials and Instrumentation
The compounds CoCl2.2H2O, NiCl2.6H2O, MnCl2.6H2O are obtained from BDH. Whereas dppm, dppp, dppf, were commercially available and obtained from Yacoo chem. China. Dithizone from Sigma Aldrich Company.
Micro elemental analyses were obtained using EuroEA 3000 instrument. The infra-red spectra in range (4000-400 cm-1) were measured as potassium bromide discs on Shimadzu, FT-IR spectroscopy Mod IR Affinity-1CE spectrophotometer. The 1H-NMR spectra were recorded on a Bruker 400 MHZ Ultra-shied. The electronic transition spectra were taken by using AE-UV1609 (UK) CO., LTD Shimadzu, in the range (200-800) nm. The magnetic moment values of the synthesized complexes were measured at 25°C using Bruker Magnet BM6. The metal analyses were obtained by using a Py-Unicm SP9 atomic absorption spectrophotometer. The molar conductivities were recorded using a pH/Conductivity/Meter 901 (0.93 cell constant) (Turkey). Melting points of the complexes were carried out using Melting Point-MPD-100 Pixel Technology CO., Limited apparatus.
Human breast cancer cell line CAL5 was maintained by minimum essential media (MEM) (US Biological, Salem, MA, USA) afforded with 10% fetal calf serum, penicillin and streptomycin at 37°C. Rat Embryo Fibroblast (REF) cells were kept in RPMI-1640 (US Biological, Salem, MA, USA) gained by penicillin/streptomycin and 10% fetal Bovine serum (Capricorn scientific, Germany). All cell lines were obtained from cell culture research lab, Iraqi Biotechnology Company, IRAQ Biotech (Baghdad, Iraq).
Synthesis of [Co (Hdz) (k2-dppf)] Cl (1)
The solutions of a (0.0256 g, 0.1 mmol) of H2dz in 10 cm3 of methanol, (0.1 mmol, 0.0273g) of CoCl2.6H2O in a10 cm3 of methanol) and (0.0554 g, 0.1mmol of 1,1′-bis (diphenylphosphine) ferrocene (dppf) were mixed and refluxed at 55°C for 2 hrs. to give brown colored precipitate and the solutions was filtered and finally it was dried in an open air. Yield= 76%, d.p= 288°C. Anal. Calc.% for C47 H41 Cl N4 P2 Co S Fe:C, 62.30; H, 4.56; N, 6.18, S,3.54, Co, 6.50,Cl,3.91. Found %: C, 61.95; H, 4.62; N, 6.48, S; 3.85, Co; 6.73,Cl, 4.01. 1H-NMR (d6-DMSO) δ:7.78(4H,d) ,7.42( 4H,t ), 7.28 – 7.38(mH), 11.61(1H). The proposed geometrical structure is shown in Figure 1.
Synthesis of [Ni (Hdz)(k2-dppf)]Cl (2)
The solutions of a (0.0256 g, 0.1 mmol)of H2dz in 10 cm3 of methanol,(0.1 mmol, 0.0273g) of NiCl2.6H2O in 10 cm3 of methanol) and (0.0554 g, 0.1mmol of 1,1′-bis(diphenyl phosphine) ferrocene (dppf) were mixed and refluxed at 55ºC for 2 h. to give red-brown colored precipitate and the solutions was filtered and finally it was dried in an open air. Yield= 72%, d.p= 265°C. Anal. Calc. % for C47 H41 Cl N4 P2Ni S Fe: C, 62.32; H, 4.56; N, 6.19, S, 3.54, Ni, 6.48, Cl,3,91. Found %: C, 61.83.7; H, 4.72; N, 5.94, S; 2.89,Ni; 6.93, Cl, 3.98. The proposed geometrical structureis is shown in Figure 1.
![Figure 1: The suggested Geometrical Shapes of, [M (Hdz)(k2-dppf)]Cl Complex.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig1-150x150.jpg) |
Figure 1: The suggested Geometrical Shapes of, [M (Hdz)(k2-dppf)]Cl Complex. |
Synthesis of [Ni (Hdz)(k2-dppe)]Cl (3)
The solutions of (0.0256 g, 0.1 mmol) of H2dz in 10 cm3 of methanol, (0.1 mmol, 0.0273g) of NiCl2.6H2O in 10 cm3 of methanol and (0.0398 g, 0.1mmol of 1,2-bis(diphenylphosphino) ethane (dppe) were mixed and refluxed at 55 Cº for 2 h. to give violet colored precipitate and the solutions was filtered and finally it was dried in an open air. Yield= 78, d.p= 198°C. Calc.% for C47 H41 Cl N4 P2Ni S:C, 66.41; H, 4.86; N, 6.59, S, 3.77, Ni, 6.90,Cl,4.71, Found %: C, 66.85; H, 4.92; N, 6.84, S; 3.87, Ni; 7.03,Cl, 4.83. 1H-NMR (d6-DMSO) δ:7.82(4H,d), 7.46 (4H,t), (mH)7.36 – 7.44. The proposed geometrical structure is shown in Figure 2.
![Figure 2: The Proposed Geometrical Structure of, [Ni(Hdz)(k2-dppe)]Cl Complex.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig2-150x150.jpg) |
Figure 2: The Proposed Geometrical Structure of, [Ni(Hdz)(k2-dppe)]Cl Complex. |
Synthesis of [Mn(Hdz)(k2-dppf)]Cl (4)
The solutions of (0.0256 g, 0.1 mmol) of H2dz in 10 cm3 of methanol, (0.1 mmol, 0.0233g) of MnCl2.6H2O in 10 cm3 of methanol) and (0.0554 g, 0.1mmol of 1,1′-bis(diphenyl phosphine) ferrocene (dppf) were mixed and refluxed at 55ºC for 2 h. to give brown colored precipitate and the solutions was filtered and finally it was dried in an open air. Yield=81%, d.p= 238°C. Anal. Calc. % for C47 H41 Cl N4 P2 Mn S Fe:C, 62.58; H, 4.58; N, 6.21, S, 3.55, Mn, 6.09, Cl, 3,93. Found %: C, 62.87, H, 4.82; N, 6.53, S; 3.88, Mn; 6.27, Cl.4.11. The proposed geometrical structure is shown in Figure 1.
Results and Discussions
The complexes were prepared by one pot- reaction (Scheme 3). This reaction consist of mixing of the solutions of H2dz ligand in methanol, MCl2.6H2O where M= Ni, Mn and Co in methanol and 1,1′-bis(diphenyl phosphine) ferrocene (dppf) or 1,2-bis(diphenylphosphino) ethane (dppe) and refluxed at 55ºC for 2 h. The properties of complexes are tabulated in Table (1), the proposed structural formula are approved by IR, 1H-NMR, UV-Visible spectra, CHNS analysis, magnetic susceptibility and molar conductivity measurement. The synthesized complexes have biological activity against breast cancer IC50 cell line that have a significant differences from cisplatin.
 |
Scheme 3: one pot reaction for the synthesis of complexes. |
Table 1: physical characteristics of synthesized metal Complexes.
| Complex | Formula | Yield% | d.P(°C) | Color |
| [Co(Hdz)(k2-dppf)]Cl | C47 H41Cl N4 P2 Co S Fe | 76 | 288 | Brown |
| [Ni(Hdz)(k2-dppf)]Cl | C47 H41Cl N4 P2 Ni S Fe | 72 | 265 | Red-Brown |
| [Ni(Hdz)(k2-dppe)]Cl | C47 H41Cl N4 P2 Ni S | 78 | 198 | Violet |
| [Mn(Hdz)(k2-dppf)]Cl | C47 H41Cl N4 P2Mn S Fe | 81 | 238 | Brown |
IR Spectral Studies
The IR spectra of complexes exhibited a medium intensity peaks in the range 423 – 432 cm-1 these bands are related to ν(M-N), The a bands between 507 – 510 cm-1 are attributed to ν(P-Ph) and bands in (744 – 750) cm-1 region are related to ν(C-S) and many other bands that shown in the9 Table 2.
Table 2: Selected IR stretching vibration bands in (cm-1) for the prepared complexes.
| Complex | v(N-H) | v(C-H) arom. | v(C-H) aliph. | ν(N=N) | ν(C=N) | ν(C-S) | ѵ(P-Ph) | ѵ(M-N) |
| 1 | 3437w | 3053w | – | 1479m | 1600m | 746m | 510m | 423w |
| 2 | 3439w | 3053w | – | 1463m | 1591m | 750s | 508w | 428w |
| 3 | 3437w | 3029w | 3952w | 1496m | 1593m | 744m | 507w | 432w |
| 4 | 3410w | 3103w | – | 1489m | 1602m | 752s | 515w | 425w |
1H-NMR Spectra
The 1H-NMR spectra for, [Ni(Hdz)(k2-dppf)]Cl (2) and, [Ni(Hdz)(k2-dppe)]Cl (3) Complexes showed many signals which attributed to aromatic rings10. See Table 3.
Table 3: 1H-NMR signal for [Ni(Hdz)(k2-dppf)]Cl and [Ni(Hdz)(k2-dppe)]Cl
| 4 protons | ||||
| Complex | Doublet (d) | Triplet (t) | Multiple protons | N-H |
| [Ni(Hdz)(k2-dppf)]Cl | 7.78 | 7.42 | 7.28 – 7.38 | 11.61 |
| [Ni(Hdz)(k2-dppe)]Cl | 7.82 | 7.46 | 7.36 – 7.44 | – |
Electronic Spectra
The electronic transition spectra in 10-3 M in methanol (Table 4) gave two peaks of complex (1), at ( 1764, 30674 )cm-1, which are assigned to the 4A2→ 1T1(P), and charge transfer (C.T) transitions respectively, which are indicated a tetrahedral geometry.11 The spectrum of complex (2), exhibited two transitions at 15974 cm-1, and 29411 cm-1 which are related to the 3T1→ 1T1(p) and C.T respectively, these bands indicated tetrahedral geometry.12 The spectrum of complex (3), gives two peaks at (16155, 30674) cm-1 which are assigned to 3T1→ 1T1(p) and C.T transitions respectively. These transitions value are approved the tetrahedral geometry.13 The spectrum of complex(4), exhibits three peaks at (15384,22371 , 31250) cm-1. These transitions values approved a tetrahedral geometry.14
Magnetic Moments
The magnetic moments of complexes [Co (Hdz) (k2-dppf)] (1), [Ni (Hdz)(k2-dppf)]Cl (2), [Ni (Hdz)(k2-dppe)]Cl (3) and [Mn(Hdz)(k2-dppf)]Cl (4) are between (4.0- 5.20) B.M. (Table 4). These data are agreed with the tetrahedral geometrical complexes. The Cl% has been determined after digestion 0.1g of the complexes in (3-5) cm3 of 6M of HNO3 and their titrations against 5% AgNO3.16
Conductivity Measurement
The molar conductivity of complexes [Co (Hdz) (k2-dppf)] (1), [Ni (Hdz)(k2-dppf)]Cl (2), [Ni (Hdz)(k2-dppe)]Cl (3) and [Mn(Hdz)(k2-dppf)]Cl (4) in methanol are 46, 22, 28 and 60 (Ω-1 cm2 mol-1) respectively indicating that they are electrolytes complexes in the 1:1 ratio.17 See Table 4.
Table 4: Electronic spectra, magnetic moments and molar conductivities of Complexes.
| Complex | λmax (nm)/ | assignments | μeff (BM)a | ʌm (cm2. Ohm-1. Mol-1)/ |
| Methanol | Methanol | |||
| 1 | 586 | 4A2 → 4T1(P) | 4 | 46 |
| 326 | C.T | Td | ||
| 2 | 273 | C.T | 4.2 | 22 |
| 626 | 3T1 → 3T1(p) | Td | ||
| 3 | 326 | C.T | 4.22 | 28 |
| 619 | 3T1 → 3T1(p) | Td | ||
| 4 | 320 | C.T | ||
| 477 | 6A1 → 6T2(G) | 5.2 | 60 | |
| 650 | 6A1 → 6T1(G) | Td |
Methylthiazolyltetrazolium (MTT) Solution
Methylthiazolyltetrazolium (MTT) (0.2g) (Bio-world, USA) was dissolved in 100 ml of PBS in order to prepare a 2 mg/ml concentration of the dye. The resulted solution was filtered by 0.2 µm syringe filter to remove any produced blue formazan, and then kept in sterile, dark, screw-capped bottles at 4°C. The solution was used within no longer than two weeks of preparation.
Cytotoxicity Essay
MTT cell viability assay was joined on 96-well plates (Santacruz Biotechnology, USA), CAL5 cells were planted at 10000 cells/well, 200μl of cells in growing medium were added to each well of a sterile 96-well micro titration plate. The plates were placed with a self-adhesive film, then put in incubator at 37°C.
The achievement of monolayer has been obtained after 24h the medium was removed and serial dilutions of the extract were added to the wells at 2-fold serial dilutions (1000ug, 500ug, 250ug and 125ug). Triplicates were used for each. Control cells treated with Serum Free Media only, and then the plates were put again in incubator at 37°C for 72 h (see Table 5).
Table 5: Cytotoxic activities of the prepared complexes on the CAL5 cell line.
T/Ccorr. [%]b+(S.D)a
|
Complex |
1000 µg |
500 µg |
250 µg |
125 µg |
|
1 |
20.01± 0.007 |
12.05 ± 0.017 | 10.29 ± 0.009 | 8.18 ± 0,06 |
|
2 |
24.10± 0.013 | 20.89 ± 0.011 | 18.11 ± 0.51 | 17.45 ± 0.016 |
|
3 |
25.56± 0,0051 | 15.85 ± 0.000 | 14.75 ± 0.0035 | 13.51 ± 0.0014 |
|
4 |
28.12 ± 0.0056 | 10.05 ± 0.023 | 10.29± 0.0097 | 8.18 ± 0.0023 |
|
MLc |
28.12 ±0.018 | 31.48 ±0.015 | 27.17± 0.01 | 24.76 ± 0.096 |
|
Cisplatin |
34.91 ± 0.094 | 28.56 ± 0.00 | 27.83 ± 0.011 | 26.07 ± 0.05 |
aS.D standard Deviation, bCytocidal effect, cML Mixed ligand
Cell viability was recorded after 72 h of exposure by removing the medium, addition of 28 µl of 2 mg/ml of MTT solution (Bio-World, USA) and then placing in incubator for 1.5h. at 37°C. The MTT solution was removed then the formed crystals in the wells was solubilized by the addition of 50 µl of DMSO (Santacruz Biotechnology, USA) sequenced by 37°C incubation for 15 min with shaking. The absorbence was recorded on a micro plate reader (Biochrom, UK) at 584 nm .The process was carried out in 5 replicates.
The mentioned procedure leads to calculation of: 1- % of cell viability or % of cell proliferation (PR) = mean of treatment / mean of control. 2- Lowest concentration that kills 50% of cells (CAL5).
Cytotoxicity Results
The protocol described was according to.18,19 Human Breast cancer cell line CAL5 was used for evaluation anticancer activities of prepared complexes using cisplatin as a reference, the results indicated that the Co(II) complex exhibited the highest cell viability Table 5. Table 6 showed the cytocidal effects of complexes were exhibited as adjusted T/C regarding to the applicable relationship (1).20
T/Ccorr.[%] = [(T-Co)/(C-Co)] x100———-(1)
Cytocidal effect [%] =[(Co –T)/Co]x100 ——-(2);
if T< Co. (Where T: mean of the cell absorbance C: mean of the control Co: The absorbance of cell when t=0).The significant differences from cisplatin have been observed for all treatment complexes especially for [Co(Hdz)(K2-dppf)]Cl and [Ni(Hdz)(K2-dppe)]Cl, Table 6 ,Figures .(3- 8).
Table 6:% Cell viability of selected compounds versus IC50 concentration.
|
Compounds |
125µg | 250µg | 500µg | 1000µg |
| [Co(Hdz)(k2-dppf)]Cl | 91.8 | 89.7 | 87.94 | 79.5 |
| [Ni(Hdz)(k2-dppf)]Cl | 82.54 | 81.88 | 79.10 | 75.96 |
| Ni(Hdz)(k2-dppe)]Cl | 89.55 | 88.10 | 80.42 | 75.96 |
| [Mn(Hdz)(k2-dppf)]Cl | 86.4 | 85.24 | 84.10 | 74.40 |
| Mixed ligand(ML) | 75.20 | 72.80 | 68.50 | 71.80 |
| Cisplatin | 73.90 | 72.00 | 71.43 | 65.00 |
![Figure 3: Breast cancer cells 72 hours MTT assay for [Co (H2dz)(dppf) ]Cl.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig3-150x150.jpg) |
Figure 3: Breast cancer cells 72 hours MTT assay for [Co (H2dz)(dppf) ]Cl. |
![Figure 4: Breast cancer cells 72 hours MTT assay for [Ni(H2dz)(dppe)]Cl.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig4-150x150.jpg) |
Figure 4: Breast cancer cells 72 hours MTT assay for [Ni(H2dz)(dppe)]Cl. |
![Figure 5: Breast cancer cells 72 hours MTT assay for [Ni(H2dz)(dppf) ]Cl.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig5-150x150.jpg) |
Figure 5: Breast cancer cells 72 hours MTT assay for [Ni(H2dz)(dppf) ]Cl. |
![Figure 6: Breast cancer cells 72 hours MTT assay for [Mn(Hdz)(dppf)]Cl.](http://www.orientjchem.org/wp-content/uploads/2018/08/Vol34No4_Syn_Hik_fig6-150x150.jpg) |
Figure 6: Breast cancer cells 72 hours MTT assay for [Mn(Hdz)(dppf)]Cl. |
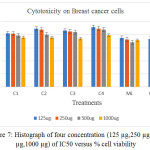 |
Figure 7: Histograph of four concentration (125 µg,250 µg, 500 µg,1000 µg) of IC50 versus % cell viability. |
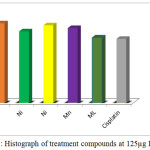 |
Figure 8: Histograph of treatment compounds at 125µg IC50 cell |
Conclusion
All complexes have been synthesized from one- pot reaction. The characterization of the structure of the complexes shows a tetrahedral geometry around the metal center, Figure (1,2). Cytocidal effect study versus different concentrations of breast cancer IC50 cell line showed significant differences from cisplatin.
Acknowledgement
The author is acknowledger to the Department of Chemistry, College of Education, at Salahaddin University for supporting this work. Nelson Mandela Metropolitan University, Faculty of Science for their helps. Baghdad, Anticancer Research Center for anticancer evaluation of the complexes.
References
- Segapelo T., Guzei I., SpencerL.C. Vanezyl W.E., Darkawa, J. Inorganica Chimica Acta. 2009, 362, 3314-3324.
CrossRef - Wheat N.J.,Walker S. Craiy, G.E. Oun, R. Dalton Trans.2010, 39, 8113-8127.
- Meijer C. , Mulder, N. H.,Timmer-Brocha H.,Sluiter W. J., Meersma, G.J.,deVries, E.G. Cancer Res.1992, 52, 6858.
- Fokkema E., Groen, H. J., Helder M. N., Devries E.G. Meijer, C. Biochem. Pharmacol 2002, 63, 1989-1996.
CrossRef - Farrell, N. Comprehensive Coordination Chemistry II2003, 9, 809-840.
- Desoize B. Anticancer Research 2004, 24, 1529-1544.
- Jack M., Chaveng P., Allan H. WJ. Chem. Soc. Dalton Trans.1983, 1009-1113.
- Ricordi C. ActaDiabetol. Lat. 1990, 27, 185-195.
CrossRef - Ali K. O. Mohamad H. A. Gerber T.I., Hosten, E. C. Orient. J. Chem.2017, 33(2), 584-592.
- Mukiza J. Gerbe T.I., Hosten E.C. Inorg. Chem. Commun. 2014, 47, 164-167.
CrossRef - Ispir E. Dyes and Pigments2009, 82 (1), 13–19.
CrossRef - Tümer M.,Deliggönül, N., Gölcü A., Akgün, E., Dolaz M. Trans. Met. Chem. 2006, 31, 1-12.
CrossRef - Singh M.,Pandy A. K.,Butcher R. J.,Singh N. K.Polyhedron2009, 28, 461-466.
CrossRef - Shaker S. A., Khaledi, H., Cheach, S. Arab. J. Chem.2016, 9, S1943-S1950.
CrossRef - Nitha L. P., Aswathy R. M., Kumari N. E., MohananK B. S.. SpectrochimicaActa A: Molecular and Bimolecular Spectroscopy, 2014, 118, 154–161.
CrossRef - Vogel A. I., A text book of quantitative inorganic analysis including elementary instrumental analysis, 3rded, Wiely, 1967, 461.
- AL-Ghazawi F. A. IJSER2014, 5, 554-558.
- Al-Shammari, A. M.Salman, Saihood M. I., Yaseen, M. I., Raed Y. D., Shaker N. Y. K., Duiach, H. K.Biomedicines2016, 4(1), 3-10.
CrossRef - Al-Samadi M., Freihat A., Abu Arqub O., Shawagfeh N. , J. Comput. & Anal. Appl.2015, 19,713-724.
- Utku S., Gumus F., Karaoglu T., Ozgul A. J. Fac. Pharm, Ankara 2007,36 (1) 21 – 30.

This work is licensed under a Creative Commons Attribution 4.0 International License.










