Responses of Oryza sativa L.Towards Azo Functionalised Schiff Base Cu(II) Complexes and CuSO4: A Comparative Biochemical Study
Kaushik Acharjee1, Jayanwita Sarkar2, Prahlad Deb3, Usha Chackraborty2 and Biswajit Sinha1
1Department of Chemistry, University of North Bengal, Darjeeling, West Bengal, India-734 013.
2Department of Botany, University of North Bengal, Darjeeling, West Bengal, India- 734013.
3Division of Horticulture, Institute of Agriculture, Visva-Bharati Univrsity, Sriniketan, West Bengal, India-731236.
Corresponding Author E-mail: kashacharjee@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/3404037
Article Received on : 20-05-2018
Article Accepted on : 17-07-2018
Article Published : 25 Aug 2018
Azo fuctionalised Schiff base ligands having N2O2 donor binding sites are capable of forming metal complexes and can be used as potential plant micronutrient supplier. Two different ligands and their copper complexes were synthesised by conventional protocols and then characterised by both spectroscopic and elemental analysis. Investigations were done by taking rice seeds as plant material. Various growth and biochemical parameters were monitored by taking different concentrations of CuSO4, prepared ligands and their Cu(II) complexes. Analysis of various biochemical results reveal that Schiff base Cu(II) complexes have less toxic effects than copper sulphate on rice seedlings and thus facilitates better tolerance to copper toxicity than copper sulphate.
KEYWORDS:Azomethine group; Copper tolerance; Cu(II) chelates; Electrolyte leakage; Plant growth; Rice seeds
Download this article as:| Copy the following to cite this article: Acharjee K, Sarkar J, Deb P, Chackraborty U, Sinha B. Responses of Oryza sativa L.Towards Azo Functionalised Schiff Base Cu(II) Complexes and CuSO4: A Comparative Biochemical Study. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Acharjee K, Sarkar J, Deb P, Chackraborty U, Sinha B. Responses of Oryza sativa L.Towards Azo Functionalised Schiff Base Cu(II) Complexes and CuSO4: A Comparative Biochemical Study. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48728 |
Introduction
Copper having the most stable oxidation state of +2, is one of the important redox active transition metal for plants.1 The optimum content of Cu2 + in plant tissue is around 10µg g-1 dry weight as reported by Baker and Senef in 1995.2 It serves as a component of different enzymes (plastocyanin, cytochrome oxidase etc.) which are mainly associated in electron transfer chain. It acts as cofactors in several enzymes such as Cu/Zn superoxide dismutase, laccase, cytochrome C oxidase.3 It is required in the pathway of photosynthesis, respiration and associated in carbohydrate and protein metabolism.4 In plants, Cu is necessary for cytosol, endoplasmic reticulum, chloroplast stroma, thylakoid lumen etc. To make Cu available in plants generally Cu contained fertilisers of inorganic origin (mainly CuSO4) are used. These types of compounds being ionic in nature are responsible for the alteration of the pH of the medium.5,6 Owing to that more emphasis is given to metal chelates which are less reactive but can solve the deficiency problem for longer period of time.7,8 Inspired by the facts we have synthesised two azo functionalised Schiff base ligands and their Cu2+ complexes. The important feature of these ligands and complexes is that they contain azomethine group (RHC=N-R/, The R and R/ are various alkyl or aryl groups) in which the nitrogen atom is sp2 hybridised and the presence of a lone pair make it a good donor especially when one or more donor atoms are present adjacent to it. Complexes of these types draw the attention because of their catalytic power in oxygen atom transfer reaction, mediating organic redox reactions.9-12 In our study, we have evaluated the responses of rice seedlings to metal toxicity while being exposed to Copper Sulphate (CuSO4) and two Copper Schiff base complexes and the results are compared in terms of pigments, biochemical components, osmolyte accumulation, oxidative stress markers and overall tolerance level of plants.
Materials and Methods
Synthesis of ligands and complexes
0.1 mol of aniline (A.R grade, procured from S. D. Fine Chemicals, India) was dissolved first in concentrated HCl without any further purification. 8g of analytical grade NaNO2 (in water) was then added to aniline solution drop wise with constant stirring for 1 hour keeping the reaction temperature throughout 00C. A solution of 0.1 mol salicyladehyde (A.R grade, procured from S. D. Fine Chemicals, India of purity level > 99%), sodium carbonate (36g) and water was added drop wise in the above mixture with stirring. The reagents were allowed to react for 5 hours at 00C. After the completion of reaction the light red precipitate of 4-(Benzeneazo)salicylaldehyde is fitered off and recrystalised in ethanol. The melting point was 1180C with yield of 80%. Two different diamines (ethylenediamine and o-phenylene diamine) were treated with the azo compound synthesized earlier in 1:2 ratios in ethanol medium for 3 hours at ambient temperature resulting two ligands. Ligands were washed and recrystalised in ethanol medium having yield 70%. The prepared two ligands were alternatively refluxed with Cu(AcO)2.H2O (A.R grade, purity level > 99%) in ethanol medium in 1:1 ratio for 2 hours at ambient temperature resulting two Cu complexes. They were filtered, washed and recrystalised and the yield was 70% almost. The two ligands L1 and L2 { [N,N/-bis[4-(benzeneazo) salicylaldehyde]ethylenediamine and [N,N/-bis[4-(benzeneazo) salicylaldehyde]-o-phenylenediamine respectively}and two complexes C1 and C2 {[N,N/-bis[4-(benzeneazo) salicylaldehyde]ethylenediamine Copper (II) and [N,N/-bis[4-(benzeneazo) salicylaldehyde]-o-phenylenediamine Copper(II) respectively} were characterised by elemental analysis (Perkin Elmer 2400 CHN analyzer), IR spectroscopy (PerkinElmer FT/IR-RX 1 spectrometer), conductance measurement (Shanghai DDS-11A conductivity apparatus at 250C) and electronic spectra (Perkin-Elmer Lambda 7 spectrophotometer). All results are quite identical with the literature.13
Treatment with ligands and complexes
To investigate the effects of prepared complexes, ligands and CuSO4, rice seeds were selected as plant material. Grains of rice (Oryza sativa L.) were surface sterilized with sodium hypochlorite (0.1%) for 1 minute, and then they were washed 3 times with distilled water14 and soaked for 12 hours with various concentrations (10, 50, 100, 200 ppm) of CuSO4, L1, L2, C1 and C2 along with a control (no treatment of micronutrients). After soaking was done the seeds were allowed to germinate on sterile petri plates. From each treatment we had chosen twenty germinated seeds which were transferred to plastic pots containing garden soil, well rotten cow dung and sand in 1:1:1 ratio under controlled conditions with a 8 hour light period at 28-35oC day/night temperature and 65-75 % relative humidity. 45 days old seedlings were subjected to biochemical analysis.
Growth parameters
To determine fresh weight, the harvested plants were rinsed with distilled water and blotted on paper towels before being weighed. Dry matter yields of the seedlings were determined after drying the seedlings in an oven at 80ºC to a constant weight. Relative water content (RWC) was measured according to the protocol described by Farooqui et al.15
Copper Tolerance Index
Copper tolerance index (TI) was calculated as the quotient of the dry weight of plants grown under copper treated and control conditions16 according to this formula:
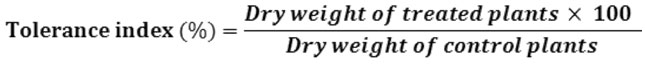
Electrolyte Leakage
Ion leakage was measured as electrical conductivity (EC %) according to Yan et al.17 The percentage of electrolyte leakage was calculated according to this formula:
![]()
Where A1 and A2 are the electrolyte conductivities measured before and after boiling, respectively.
Determination of free amino acids and proline
Free amino acids were detected according to the method of Lee and Takahashi.18 Proline content was determined by ninhydrin method19.
Lipid Peroxidation
It was measured as the content of malonyldialdehyde (MDA) using the thiobarbioturic method of Heath and Packer.20
H2O2 Content
H2O2 levels in the leaves were estimated according to Jena and Choudhuri21 with minor modifications. H2O2 levels were calculated using extinction coefficient 0.28 µmol–1 cm–1.
Chlorophyll Content
Chlorophyll was estimated according to the method of Harborne.22Estimation of chlorophyll was done by measuring the absorbance at 645 nm and 663 nm respectively in a UV-Vis spectrophotometer against a blank of 80% acetone and calculated using the formula as given by Arnon.23
Total chlorophyll = (20.2 A645 + 8.02 A663) mg (g tissue)-1 F.W
Carotenoid Content
The extraction and quantification of carotenoids was completed by following the method of Litchtenthaler.24 The absorbance was then assessed at 480 nm, 645 nm and 663 nm in UV-Vis spectrophotometer and the carotenoid content was estimated using standard formula:
A480 – (0.114 × A663) – 0.638 (A645) µg (g tissue)-1 F.W
Statistical Analysis
Data were analysed by using Standard Error and LSD tests at P ≤ 0.05 probability level using IBM SPSS statistics 21 software
Results and Discussion
Characterisation of the prepared ligands and Cu (II) complexes
The analytical data and corresponding Infrared spectra of the prepared ligands and the corresponding Cu (II) complexes are almost identical with the literature13 and are provided in the supplementary (Table 1, Table 2, Table 3 and Scheme of reaction). The prepared two ligands show characteristic bands in the region of 1280 cm-1(for O-H bon), 1605-1635cm-1(for C=N bond vibration). A broad band in the region of 2800-2700cm-1 signifies a strong hydrogen bonding between the hydroxyl hydrogen and nitrogen atom. For the prepared Cu complexes characteristic ῡ(C=N), ῡ(phenolic C-O), ῡ(Cu-N), ῡ(Cu-O) were observed. All Infrared spectra exhibit parity with literature.
The ligand L2 shows 3 main peaks 254.0, 318.0 and 368.5 nm in the electronic spectrum. The first and second peaks are due to benzene 14Ï€”> – 14Ï€”> * and imino 14Ï€”> – 14Ï€”> *transitions where as the third peak is due to 14n”> – 14Ï€”> * transition. For C2 the third peak is shifted towards longer wave length due to donation of lone pair to Cu. The ligand L1 shows mainly two peaks and they are 270.5 and 362.5 nm (first one for 14 Ï€”> – 14Ï€”> * and second one for 14n”> – 14Ï€”> *). In C1 the 14 n”> – 14Ï€”> * peak shifted to 396 nm.
Growth and tolerance to different copper complexes
Cu2+ can hamper growth and development of seedlings by causing damage to epidermal cells and cell membranes.25 The outcomes reveal that plants treated with C1 and C2 complex were able to retain a higher percentage of fresh weight, dry weight and relative water content (RWC) than CuSO4 treated plants with increasing concentration suggesting less toxicity of Schiff base complexes (Figure 1, Figure 2 and Figure 3).
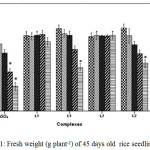 |
Figure 1: Fresh weight (g plant-1) of 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level |
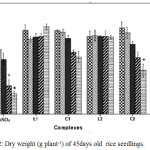 |
Figure 2: Dry weight (g plant-1) of 45days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
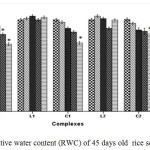 |
Figure 3: Relative water content (RWC) of 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. Click here to View figure |
For instance at 100 and 200 ppm CuSO4 significantly reduces RWC by 22.99% where as C1 and C2 complex reduce only by 12.01% and by 12.34% respectively over control. Greater negative impact of CuSO4 than the schiff base complexes on seedlings was further approved by Tolerance index. TI for C1 and C2 complex treated plants appeared to be significantly higher than that of CuSO4 treated plants (Figure 4).
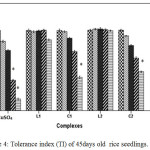 |
Figure 4: Tolerance index (TI) of 45days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. Click here to View figure |
Negative effect of CuSO4 was also reported by Azooz et al.26 in wheat seedlings. Verma et al.27 reported application of copper at lesser concentrations enhanced the plant’s dry biomass, while excess of copper reduced the biomass production of these plants.
Membrane damage and ion leakage
Copper can distress the membrane permeability by oxidation of membrane lipids which can be accessed from the increase of MDA, one of the lipid peroxidation products. Data obtained from this study revealed copper induced membrane damage which is expressed in terms of electrolyte leakage, lipid peroxidation enhanced significantly in CuSO4 treated plant with rising concentration but in case of Schiff base complex treated plants malonyldialdehyde accumulation as well as electrolyte leakage were less as compared to CuSO4 treated plants in relation to control indicating comparatively less membrane damage in Schiff base complex treated plants.
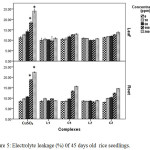 |
Figure 5: Electrolyte leakage (%) 0f 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
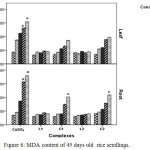 |
Figure 6: MDA content of 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level |
The outcomes showed that the MDA content in leaf (Figure 6) and electrolyte leakage in leaf and root (Figure 5) of Schiff base Cu(II) complex treated plants increase with increasing dose but did not echo any significant changes. But in case of CuSO4 treated plants ion leakage and oxidation of membrane lipid enhanced drastically at 100 and 200 ppm level in relation to control plants.
Reactive oxygen species induced plasma membrane damage increase MDA and ion leakage at the higher levels of Cu2+.28 Hydrogen peroxide [one form of reactive oxygen species (ROS)] accumulation is minimum in case of schiff base Cu(II) complex treated plants than in CuSO4 treated plants (Figure 7).
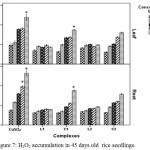 |
Figure 7: H2O2 accumulation in 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
In copper sulphate treated plants H2O2 increased 1.35 fold at 200 ppm where in Schiff base complex treated plants especially in case of C2 complex H2O2 accumulation was less (0.62 fold) in relation to control. This observation appears to be narrated the redox-active character of Cu2+ resulting in creation of extremely reactive hydroxyl radicals.29 These results suggest that Schiff base Cu(II) treated plants had better ability to tolerate Cu2+ stress. The significant increase of MDA in plants, exposed to CuSO4 indicated that increase of lipid peroxidation in Cu-treated plants led to disorder of plasma membranes on contrary plasma membrane damage was less in case of Schiff base Cu (II) complexes treated plants.
Total free amino acids and proline
Accumulations of total free amino acids (Figure 8) were significantly increased in root and leaf tissue at higher CuSO4 concentrations. However, low CuSO4 up to 50 ppm had non-significant enhancement on total free amino acids. The highest increase in free amino acids in case of CuSO4 treatment was noticed at 100 and 200 ppm in both leaf and root tissue. On contrary both the Schiff base Cu(II) complex treated plants showed insignificant enhancement of amino acids.
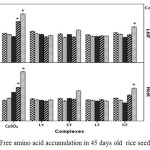 |
Figure 8: Free amino acid accumulation in 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
Al-Hakimi and Hamada,30 in their study, suggested that free amino acids content enhances in plant tissues upon Cu2+ exposure. In agreement amino acids are looked upon as key player in metal chelation through which plant detoxify alleviate heavy metal stress.31 Therefore, it might be suggested that plants experiencing higher amount of copper induced oxidative stress can accumulate greater amount of free amino acid to alleviate oxidative stress. In that scenario copper Schiff base complexes are proved to be less toxic than CuSO4.
Proline accumulation in plant tissue which is an indicator of oxidative stress32 increased in all treatments. In CuSO4 treated plants proline accumulation was maximum at 100 ppm and at 50 ppm in leaf and root tissue respectively. Beyond this concentration proline accumulation decreased. Decline in proline accumulation may be attributed to reduction competence of plants to withstand oxidative stress.33 Conversely, in Schiff base Cu(II) complex treated plants, proline content continued to be increased up to 200 ppm signifying lesser oxidative stress in those plants (Figure 9).
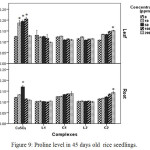 |
Figure 9: Proline level in 45 days old rice seedlings. Means ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
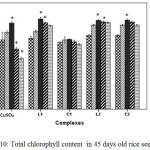 |
Figure 10: Total chlorophyll content in 45 days old rice seedlings. Mean ± SE, n = 3. * The mean difference is significant at the 0.05 level |
Carotenoid, a non enzymatic antioxidant involved in quenching of oxidizing species, participate in disrupting regular cellular functioning. In CuSO4 treated plants carotenoid content increased gradually upto 100 ppm. At 200 ppm carotenoid content reduced drastically signifying the huge toxicity of CuSO4 but on contrary in C1 and C2 treated plants carotenoid content continued to increase up to 200 ppm (Figure 11).
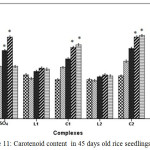 |
Figure 11: Carotenoid content in 45 days old rice seedlings. Means ± SE, n = 3. * The mean difference is significant at the 0.05 level. |
Conclusions
Offshoots of the present study reveals that Schiff base Cu(II) complexes have less toxic effects than copper sulphate on rice seedlings and provide better tolerance to copper toxicity than copper sulphate. Maximum positive impact of Schiff base complexes was found mostly at 50 ppm concentration. Though different stress marker and reactive oxygen species accumulation were less and minimum pigment damage was noticed in Schiff base Cu (II) complex treated seedlings but the optimum positive impact of these Schiff base Cu(II) complexes largely depends on dose. Beyond certain concentration these complexes may have inhibitory effects on rice. Cu(II) schiff base complexes can be used as a supplement to turn down micronutrient deficiency at the same time minimize the toxicity generated by application of different ionic form of Cu(II) may open a new direction research.
Acknowledgement
The authors are grateful to the Departmental Special Assistance Scheme under the University Grants Commission, New Delhi (SAP-DRS-III, No. 540/12/DRS/2013) for financial support. Authors (K. Acharjee and J. Sarkar) are thankful to UGC, India and Department of science and technology, India respectively for providing fellowships.
References
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156.
CrossRef
- Baker, D. E., Senef, J. P. Heavy metals in soils. Blackie Academic and Professional, 1995, 179-205.
CrossRef - Tanyolac, D., Ekmekci, Y., Unalan, S. Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere, 2007, 67, 89–98.
CrossRef - Draobzkiewicz, M., Skórzyńska-Polit, E., Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. BioMetals, 2004, 17, 379–387.
CrossRef - Thind, S., Rowell, D. Effects of algae and fertilizer nitrogen on pH, Eh and depth of aerobic soil in laboratory columns of a flooded sandy loam. Biol Fertil Soils.1999, 28, 162-168.
CrossRef - Brennan, R., Bolland, D. Residual values of soil-applied zinc fertilizer for early vegetative growth of six crop species. Aust J Exp Agric.2006, 46, 1341-1347.
CrossRef - Khoshgoftarmanesh,H., Schulin, R., Chaney, R., Daneshbakhsh, B., Afyuni. M. Micronutrient- efficient genotypes for cropyield and nutritional quality in sustainable agriculture. A review. Agron Sustain Dev. 2010, 30, 83–107.
CrossRef - Wallace, A., Wallace, A. Micronutrient uptake by leaves from foliar sprays of EDTA chelated metals. Iron nutrition and interactions in plants, 1982, 975–978.
- Butler, A., Carrano, C. Coordination chemistry of vanadium in biological systems. Coord Chem Rev. 1991, 109, 61–105.
CrossRef - Katsuki, T. Catalytic asymmetric oxidations using optically active (salen)manganese(III) complexes as catalysts. Coord. Chem. Rev. 1995, 140, 189-214.
CrossRef - Boghaei, D., Mohebbi, S. Non-symmetrical tetradentate vanadyl Schiff base complexes derived from 1,2-phenylene diamine and 1,3-naphthalene diamine as catalysts for the oxidation of cyclohexene. Tetrahedron, 2002, 58, 5357-5366.
CrossRef - Mohebbi, S., Boghaei, D., Sarvestani, A., Salimi, A. Oxovanadium(IV) complexes as homogeneous catalyst—aerobic epoxidation of olefins. Appl. Catal. A: Gen. 2005, 278, 263-267.
CrossRef - Liu, J. N., Wu, B. W., Zhang, B., Liu, Y. Synthesis and characterization of metal complexes of Cu(II), Ni(II), Zn(II), Co(II), Mn(II) and Cd(II) with tetradentate schiff bases. Turk J Chem. 2006, 30, 41-48.
- Sauer, D., Burroughs, R. Disinfection of seed surfaces with sodium hypochlorite. Phytopathology, 1986, 76, 745-749.
CrossRef - Farooqui, A., Kumar, R., Fatima, S., Sharma, S. Response of different genotype of lemon grass (Cymbopogaon flexuosus and C. pendulus) to water stress. J Plant Biol. 2000, 27, 277-282.
- Bálint, A.F., Kovács, G., Sutka, J. Copper tolerance of Aegilops, Triticum, Secale and triticale seedlings and copper and iron contents in their shoots. Acta Biol Szegediensis, 2002, 46, 77-78.
- Yan, B., Dai, Q., Liu, X., Huang, S., Wang, Z. Flooding induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil. 1996, 197, 261-268.
CrossRef - Lee, Y.P., Takanashi. T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal Biochem. 1966, 14, 71-77.
CrossRef - Bates, L.S., Waldren, R.P., Tear, L.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205-207.
CrossRef - Heath, R. L., Packer, L. Photoperoxidation in isolated chloroplasts. I: Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968, 125, 189-198.
CrossRef - Jena, S., Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot.1981, 12, 345-354.
CrossRef - Harborne, J. Phytochemical methods. Chapman and Hall International ed., London: Toppan Company Limited, 1973.
- Arnon, D. I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1-15.
CrossRef - Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350-382.
CrossRef - Xiong, Z. T., Wang, H. Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekinensis Rupr.). Environ. Toxicol. 2005, 20, 188–194.
CrossRef - Azooz, M. M., Abou-Elhamd, M. F., Al-Fredan, M. A. Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Australian journal of crop science, 2012, 6(4), 688-694.
- Verma, J. P., Singh, V., Yadav, J. Effect of copper sulphate on seed germination, plant growth and peroxidase activity of Mung bean (Vigna radiate). Inter J Bot. 2011, 7, 200-204.
CrossRef - Yin, H., Chen, Q., Yi, M. Effect of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul. 2008, 54, 45-54.
CrossRef - Pinto, E., Sigaud-Kutner, T. C. S., Leit˜ao, M. A. S., Okamoto, O. K., Morse, D., Colepicolo, P. Heavy metal-induced oxidative stress in algae. J Phycol. 2003, 39, 1008–1018.
CrossRef - Al-Hakimi, A. B. M., Hamada, A. M. Ascorbic Acid, Thiamine or Salicylic Acid Induced Changes in Some Physiological Parameters in Wheat Grown under Copper Stress. Plant Protect. Sci. 2011, 47(3), 92-108.
CrossRef - Hall, J. L. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002, 53, 1–11.
CrossRef - Fariduddin, Q., Yusuf, M., Hayat, S., Ahmad, A. Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environmental and Experimental Botany, 2009, 66, 418–424.
CrossRef - Wang, F., Zeng, B., Sun, Z., Zhu, C. Relationship between proline and Hg2+ -induced oxidative stress in a tolerant rice mutant. Arch Environ Contam Toxicol. 2009, 56, 723–731.
CrossRef - Jain, A., Poling M. D., Smith, A. P., Nagarajan, V. K., Lahner, B., Meagher, R. B., Raghothama, K. G. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 2009, 150, 1033-1049.
CrossRef - Alaoui-Sosse, B., Genet, P., Vinit-Dunand, F., Taussaint, M. L., Epran, D., Badot, P.M. Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci. 2004, 166, 1213 – 1218.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









