Preparation and Characterization of Chloride-free Alumina-Supported Platinum Catalysts
Department of Chemistry, Yogyakarta State University, Yogyakarta, DIY 55281 Indonesia.
Corresponding Author E-mail: prodjosantoso@uny.ac.id
DOI : http://dx.doi.org/10.13005/ojc/3404046
Article Received on : 12-06-2018
Article Accepted on : 19-07-2018
Article Published : 31 Jul 2018
Supported precious metal catalysts are extensively used as efficient catalysts. This kind of catalysts, particularly chloride-free catalysts, sintesized using organoplatinum compounds as precursors has attracted immense research interest compared to their parent metals due to their unique physico-chemical properties. The main objective of this research is to prepare and characterize the chloride-free alumina-supported platinum catalysts. An organometallic compound of ammonium bisoxalatoplatinate(II) hydrate was used to prepare unsupported and alumina supported platinum catalysts. A series method including IR, XRD, SEM, TEM, EDA, and XPS was used to characterize samples. The research shows that ammonium bisoxalatoplatinate(II) hydrate could be synthesized and used to prepare unsupported and alumina supported platinum free of chloride impurities.
KEYWORDS:Catalysts; Chloride-free; Platinum; XPS
Download this article as:| Copy the following to cite this article: Prodjosantoso A. K. Preparation and Characterization of Chloride-free Alumina-Supported Platinum Catalysts. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Prodjosantoso A. K. Preparation and Characterization of Chloride-free Alumina-Supported Platinum Catalysts. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=47869 |
Introduction
Organometallic compounds are widely used as catalyst precursor.1,2 A combination of impregnation of the support with a solution containing the appropriate organometallic species and heat treatment can yield metal and/or metal oxide particles uniformly distributed over the surface of the support. Unfortunately since multi-step reaction pathways are frequently involved, the preparation of particular organometallic precursor is often time consuming. A second problem is that chloride containing species are typically the most easy-prepared organometallics.3 Thus it is possible that chloride will be present in of the resulting catalysts.4 Chloride ions have been reported to be absorbed on, and hence block, the active site of metal catalysts.4–7 As such chloride ions can inhibit a number of metal catalyzed reactions.8
Removing such chlorides can be both difficult and time consuming since requires additional processing. Potassium bisoxalatometallate complexes are simple chloride-free compounds. Potassium bisoxalatoplatinate(II) is well known and easy to be prepared.9 This complex can be thermally decomposed at relatively low temperatures to the metal and/or metal oxide, and it is ideal precursors for the formation of chloride-free catalysts.1,10 The high solubility in aqueous solutions suggests it may be straight forward to deposit this compound onto high surface area supports. After heat treated, the metal and/or metal oxide crystallites dispersed over the surface of the support will be obtained. The presence of K as a product of heat treatment of potassium bisoxalatoplatinate(II) was confirmed.11,12
In general potassium is not believed enhance the catalytic activity of Pt, and there is some evidences that potassium acts as inhibitor. Fortunately the K species could be removed by washing the heat treated samples with water. However, washing heat treated K2Pt(ox)2.2H2O with water failed to remove all the potassium since in this case the insoluble oxide was formed.13,14 The structural studies of K2Pt(ox)2.2H2O demonstrate the presence of discrete Pt(ox)22- and therefore it should be possible to replace potassium with ammonium cation.15,16 The advantages should be realized by avoiding the use of K. Since the ammonium cation will be oxidized during heat treatment, subsequent washing of the Pt product should be unnecessary.
This research is to establish if chloride-free Pt catalysts could be prepared using complex of the type (NH4)2Pt(ox)2.2H2O.
Experimental and Method
Ammonium bisoxalatoplatinate(II) (NH4)2Pt(ox)2.2H2O was prepared using a modification of the method described by Schlutz et al. for Co0.83Pt(ox)2.6H2O.17 An excess of 1 M HCl was added to Ag2Pt(ox)2 (5 g, 0.008 mol) suspended in 100 mL of water. The resulting AgCl precipitate, coagulated by heating the solution, was removed by filtration to yield a clear yellow solution of H2Pt(ox)2.(NH4)2(ox) (2 g, 0.016 mol) dissolved in 20 mL of water was added to the filtrate and the resulting yellow solution was re-filtered and allowed to stand, in air, at room temperature for 2 weeks. The solution slowly turned blue and deposited yellow needle-shaped crystals of the desired product. These were collected and washed successively with cold water, alcohol and acetone and finally air dried. The preparation of alumina supported (NH4)2Pt(ox)2.2H2O was achieved by dissolving (NH4)2Pt(ox)2.2H2O (0.4964 g, 0.0013 mol) in 25 mL of hot water containing a suspension of Al2O3 (2.549 g, 0.025 mol). The volume of the solution was then reduced to about 5 mL by heating rapidly and stirred solution at near boiling point. After cooling to room temperature, the yellow solid was collected and successively washed with cold water, alcohol and acetone and finally air dried. Samples were heat treated in a muffle furnace for four hours at 950oC and 1100oC for unsupported and supported samples, respectively. Infrared (IR) spectra were recorded on a Biorad FTS-40 spectrophotometer between 400 to 4000 cm-1. Powder X-ray diffraction (XRD) patterns of the samples were collected using Cu Kα radiation (λ = 1.5405 Å) peaks, observed at 2θ range between 5oC to 90oC with step size of 0.01o per second, and recorded on a Siemens D5000 diffractometer operating the DIFFRAC 500 software. Scanning electron micrographs (SEM) were collected using a JEOL JSM6000F microscope, while ttransmission electron microscopy (TEM) studies were performed on a Phillips CM12 microscope. Energy dispersive analysis (EDA) were performed with an EDAX PV9900 system on a Phillips 505 scanning electron microscope. X-ray photoelectron spectra (XPS) were recorded on a Kratos XSAM 800 spectrometer.
Results and Discussions
Untreated unsupported and alumina supported (NH4)2Pt(ox)2.2H2O have been synthesized, and characterized using some methods. Comparison of the infrared spectra of alumina, untreated unsupported and alumina supported (NH4)2Pt(ox)2.2H2O, suggests that (NH4)2Pt(ox)2.2H2O can be deposited onto alumina without any decomposition. It is clear from the infrared spectra that the structure of the three materials is different to each other. The broad and strong bands at the region 2800-3700 cm-1 of infrared spectra of the (NH4)2Pt(ox)2.2H2O sample feature the characteristic of NH4+ group and water, belong to (NH4)2Pt(ox)2.2H2O complex (Figure 1.), as described in smectite and illite,18 NH4+ ion in zeolitic materials,19 and NH4+-smectite nanocomposites.20
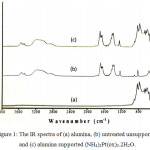 |
Figure 1: The IR spectra of (a) alumina, (b) untreated unsupported, and (c) alumina supported (NH4)2Pt(ox)2.2H2O. |
Scanning electron microscopy (SEM) was employed to study the morphology of the samples. Electron micrographs of (NH4)2Pt(ox)2.2H2O is shown in Figure 2.a. The complex forms needles being about 35 μm long. In this case there is no evidence for any minority phases. Electron micrograph of alumina supported (NH4)2Pt(ox)2.2H2O is given in Figure 2.b. The particle size of supported material is considerable smaller than the unsupported material. In this case well dispersed crystallites of oxalate complex is present on the surface the alumina particles. Chloride was not observed in the EDA of the sample studied (Figure 2.b).
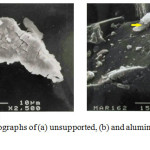 |
Figure 2: Electron micrographs of (a) unsupported, (b) and alumina supported (NH4)2Pt(ox)2.2H2O. An arrow points toward (NH4)2Pt(ox)2.2H2O on the surface of alumina. |
The EDA spectra (b) indicates the presence of the main metals i.e. Al and Pt in the alumina supported (NH4)2Pt(ox)2.2H2O sample. The XRD measurement confirms that the structure of the NH4+ salts of Pt(ox)22- is retained when it is deposited onto alumina (Figure 3). The diffraction pattern of the complex did not show any lines, except these due to alumina. There is noticeable changes in the relative intensities of the Bragg reflections in the pattern of (NH4)2Pt(ox)2.2H2O, however this is believed to be a result preferential growth of the complex on the support.
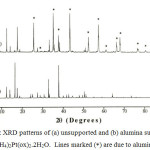 |
Figure 3: XRD patterns of (a) unsupported and (b) alumina supported (NH4)2Pt(ox)2.2H2O. Lines marked (*) are due to alumina. |
The XRD patterns of the decomposition products of alumina supported (NH4)2Pt(ox)2.2H2O formed at different temperatures are given in Figure 4. Heat treatment at 400oC produces Pt metal. Heating the sample at temperatures between 500oC to 1100oC results in crystallization of Pt. The Pt is stable even after heating to 1100oC. Unfortunately, the limitation of heat treatment unsupported sample causes the XRD pattern unable to collect.
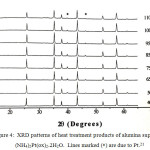 |
Figure 4: XRD patterns of heat treatment products of alumina supported (NH4)2Pt(ox)2.2H2O. Lines marked (*) are due to Pt.21 |
The XRD patterns of the decomposition products of alumina supported (NH4)2Pt(ox)2.2H2O formed at different temperatures are given in Figure 4. Heat treatment at 400oC produces Pt metal. Heating the sample at temperatures between 500oC to 1100oC results in crystallization of Pt. The Pt is stable even after heating to 1100oC. Unfortunately, the limitation of heat treatment unsupported sample causes the XRD pattern unable to collect.
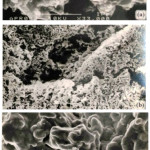 |
Figure 5: Electron micrographs of unsupported (NH4)2 Pt(ox)2.2H2O after heating at 400oC (a), 550oC (b), and 650oC (c) for 4 hours. |
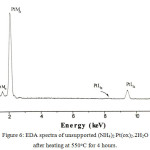 |
Figure 6: EDA spectra of unsupported (NH4)2Pt(ox)2.2H2O after heating at 550oC for 4 hours. |
SEM and EDA spectra of heat treated alumina supported (NH4)2Pt(ox)2.2H2O are shown in Figure 7 and 8. The electron micrographs of the material heated from 400 to 700oC showed a range of poorly formed crystallites to be present, whilst heating at higher temperature produces more crystalline materials of various size from 50 to 300 nm on the surface of alumina support. In comparison to the unsupported materials, it is apparent that the net-like particles did not persist on the surface of support. This is presumably has implications for sintering or reactions of the particles with the surface of support.
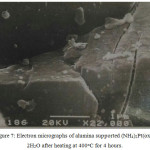 |
Figure 7: Electron micrographs of alumina supported (NH4)2Pt(ox)2.2H2O after heating at 400oC for 4 hours. |
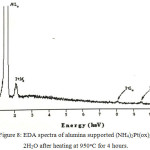 |
Figure 8: EDA spectra of alumina supported (NH4)2 Pt(ox)2.2H2O after heating at 950oC for 4 hours. |
TEM was used in order to characterize any small particles that may form on the surface of heat treated alumina supported materials. For the Pt complex a range of particle sizes were observed and the precise size of the crystallites depended on the heating conditions. For example, the heat treating the alumina supported platinum complex at 600oC produces particles with size ranging from 15 to 37 nm, which is somewhat bigger size than that found by Ivanova,21 distributed over the alumina support (Figure 9), as confirmed by EDA spectra (Figure 10) and XRD measurement (Figure 4).
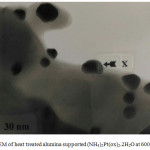 |
Figure 9: TEM of heat treated alumina supported (NH4)2Pt(ox)2.2H2O at 600oC. |
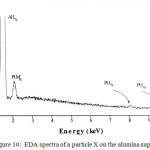 |
Figure 10: EDA spectra of a particle X on the alumina support. |
The surface composition on the heat treated alumina supported sample was studied by XPS. The survey spectra on the alumina supported sample after heating at 400 oC for 4 hours was collected (Figure 11). The spectra are dominated by strong Al and O signals from alumina support; all other lines observed are as expected. No lines due to Cl were observed.
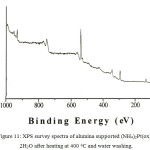 |
Figure 11: XPS survey spectra of alumina supported (NH4)2Pt(ox)2.2H2O after heating at 400oC and water washing. |
The BE value on the graphs have not been corrected for charging effect. Partial overlap of the Pt 4f and Al 2p lines complicates the analysis of the Pt 4f spectra (Figure 12). Peak fitting analysis suggest a single Pt species is present with Pt 4f7/2 BE of 70.9 eV indicating Pt(0).22 The spectra provided no evidences for the presence of any Pt oxides.
 |
Figure 12: Pt 4f spectra of alumina supported (NH4)2 Pt(ox)2.2H2O after heated at 950oC. Click here to View figure |
The BE value on the graphs have not been corrected for charging effects of 2.8 eV.
Conclusion
A series of IR, XRD, SEM, TEM, EDA and XPS studies showed that (NH4)2Pt(ox)2.2H2O can be prepared and can be used to prepare unsupported and alumina supported Pt free of chloride impurities.
Acknowlegement
I am thankful to Prof. B.J. Kennedy (Unversity of Sydney, Australia) who provided expertise that greatly assisted the research, and all of the interpretations provided in this manuscript.
References
- Thurier, C.; Doppelt, P., Coordination Chemistry Reviews, 2008, 252, 155–169.
CrossRef - Lashdaf, M.; Lahtinen, J.; Lindblad, M.; Venäläinen, T.; Krause, A.O.I., Appl. Catal. A Gen., 2004, 276, 129–137.
CrossRef - Zhao, J.; Gou, S.; Liu, F.; Sun, Y.; Gao, C., Inorg. Chem., 2013, 52, 8163–8170.
CrossRef - Gavrilović, L.; Brandin, J.; Holmen, A.; Venvik, H.J.; Myrstad, R.; Blekkan, E.A., Ind. Eng. Chem. Res., 2018, 57, 1935–1942.
CrossRef - Pendyala, V.R.R.; Jacobs, G.; Ma, W.; Sparks, D.E.; Shafer, W.D.; Khalid, S.; Xiao, Q.; Hu, Y.Davis, B.H., Catal. Letters, 2016, 146, 1858–1866.
CrossRef - Knapp, S.M.M.; Knapp, S.M.M.; Sherbow, T.J.; Ahmed, T.J.; Thiel, I.; Zakharov, L.N.; Juliette, J.J.; Tyler, D.R., J. Inorg. Organomet. Polym. Mater., 2014, 24, 145–156.
CrossRef - Wu, X.; Yu, W.; Si, Z.; Weng, D., Front. Environ. Sci. Eng., 2013, 7, 420–427.
CrossRef - Oh, H.-S.; Yang, J.H.; Costello, C.K; Wang, Y.M.; Bare, S.R.; Kung, H.H.; Kung, M.C., J. Catal., 2002, 210, 375–386.
CrossRef - Štarha, P.; Trávnícek, Z.; Popa, I., J. Inorg. Biochem., 2010, 104, 639–647.
CrossRef - Al-Jibori, S.A.; Barbooti, M.M., Al-Jibori, M.H.S.; Aziz, B.K., J. Mater. Environ. Sci., 2017, 8, 1365–1374.
- Rodríguez, D.; Sánchez, J.; Arteaga, G., Journal of Molecular Catalysis A: Chemical, 2005, 228, 309–317.
CrossRef - Zhang, Y.; Zhao, C.Y.; Liang, H.; Liu, Y., Catal. Letters, 2009, 127, 3-4.
- Batista, S.G.; Afonso, J.C., J. Hazard. Mater., 2010, 184, 717–723.
CrossRef - Jackson, S. D.; Matheson, I.M.; Naeye, M.-L.; Stair, P.C.; Sullivan, V.S.; Watson, S.R.; Webb, G., Stud. Surf. Sci. Catal., 2000, 130, 2213–2218.
CrossRef - Yamamoto, C.; Nishikawa, H.; Nihei, M.; Shiga, T.; Hedo, M.; Uwatoko, Y.; Sawa, H.; Kitagawa, H.; Taguchi, Y.; Iwasa, Y.; Oshio, H., Inorg. Chem., 2006, 45, 10270–10276.
CrossRef - Broach, R.W.; Kirchner, R.M., Microporous Mesoporous Mater., 2011, 143, 398–400.
CrossRef - Schultz, A.J.; Underhill, A.E.; Williams, J.M., Inorg. Chem., 1978, 17, 1313–1315.
CrossRef - Pironon, J.; Pelletier, M.; de Donato, P.; Mosser-Ruck, R., Clay Miner., 2003, 38, 201–211.
CrossRef - Barbera, K.; Lanzafame, P.; Perathoner, S.; Centi, G.; Migliori, M.; Aloise, A.; Giordano, G., New J. Chem., 2016, 40, 4300–4306.
CrossRef - Bobos, I., Am. Mineral., 2012, 97, 962–982.
CrossRef - Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, О.А.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I., Appl. Catal. B Environ., 2010, 97, 57–71.
CrossRef - Chastain, J., Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmeer Corporation, 1992.

This work is licensed under a Creative Commons Attribution 4.0 International License.










