Preparation and Aggregation-Induced Emission of New 1,3,5-Triazine-2,4,6-tricarboxamide with Liquid Crystal Properties
Abdulhamid Umar1,2 and Mustaffa Shamsuddin1,3
1Department of Chemistry Faculty of Science, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia.
2Adamawa State University Mubi, PMB 25 Mubi, Adamawa State, Nigeria.
3Centre for Sustainable Nanomaterials, Universiti Teknologi Malaysia, 81310 Johor Bahru, Johor, Malaysia.
Corresponding Author E-mail: mustaffa@kimia.fs.utm.my
DOI : http://dx.doi.org/10.13005/ojc/340405
Article Received on : 02-05-2018
Article Accepted on : 21-07-2018
Article Published : 16 Aug 2018
The combination of aggregation-induced emission (AIE) and liquid crystal properties generates solid-state efficient luminescent liquid crystal materials. Here in, we reported the synthesis of 1,3,5-triazine-2,4,6-tricarboxamide and utilized it as a supramolecular organic motif for the AIE-active liquid crystal material. The compound exhibits high-intensity emission maxima at 417 and 468 nm in the solid state with excitation at 254 nm, whereas it shows weak emission in the solution phase. Also, this compound behaves as liquid crystalline material and shows columnar hexagonal mesophase with endothermic peaks at 73.4oC, 185.6oC, and exothermic peaks were observed at 181.9oC and 66.1oC with focal conic fan shape texture. The thermal data showed that the compound is stable up to 200oC.
KEYWORDS:Aggregation-Induced Emission; Liquid Crystal; Luminescent; 1,3,5-Triazine-2,4,6-Tricarboxamide
Download this article as:| Copy the following to cite this article: Umar A, Shamsuddin M. Preparation and Aggregation-Induced Emission of New 1,3,5-Triazine-2,4,6-tricarboxamide with Liquid Crystal Properties. Orient J Chem 2018;34(4). |
| Copy the following to cite this URL: Umar A, Shamsuddin M. Preparation and Aggregation-Induced Emission of New 1,3,5-Triazine-2,4,6-tricarboxamide with Liquid Crystal Properties. Orient J Chem 2018;34(4). Available from: http://www.orientjchem.org/?p=48404 |
Introduction
Organic solid luminescent materials have been attracting particular attention due to their vast potential applications, in displays, optoelectronics, organic solar cells, organic light emitting diode (OLED) as well as in the field of sensing and imaging.1,2 Conventional solid organic molecules are known to exhibit strong π-π stacking interactions due to their extended π-conjugation and planar structure. This interaction promotes loss of excitation energy. Consequently, the molecules are poorly or non emissive in the solid state, even though they emit efficiently in solution. The phenomenon is called aggregation-caused quenching (ACQ). Although, many attempts have been made to overcome the ACQ effect, such as introductions of large cyclic species and dendritic wedges to fluorophores these approaches often led not only to complicated syntheses, but also undesired photophysical properties.5,6 In 2001, Tang et al. reported an unusual phenomenon with silacyclopentadine (silole) derivatives that are non emissive in solution but become highly emissive upon aggregation. The phenomenon is termed aggregation-induced emission (AIE)7. Restriction of intramolecular rotation was identified as the mechanism for AIE effect in aggregate state.8
Recently, many attention has been focused on luminescent liquid crystals (LCs) organic materials,9,10 due to their combination of light emission, supramolecular organization and broad technological applications such as organic light-emitting diodes, polarized organic lasers11 and sensors.12 In spite of the unique properties of luminescent liquid crystal molecules, their syntheses remain challenging due to difficulty incorporating emissive functional group and liquid crystal properties. Also, many conjugated molecular emitters suffer aggregation-cause quenching effect leading to the quenching of fluorescence.12 Therefore, the utilization of aggregation-induced emission would circumvent the ACQ effect. Because AIE molecules usually show strong emission in the solid or aggregate state. Although AIE-active LCs show promising prospects, the research is still rarely reported since the requirements for the LC and AIE characteristic are hard to fulfil at the same time in one single molecule. Here in, we report the first example of 1,3,5-triazine-2,4,6-tricarboxamide as an AIE-active liquid crystal (AIE-LC) bearing hydrophobic alkyl side chain with simple aromatic 1,3,5-triazine AIE core. It has been known that attaching either long alkyl/alkoxy groups to the AIE core creates light-emitting liquid crystal materials.13
Materials and methods
General
All chemicals were obtained from Aldrich and used as received without further purification unless otherwise stated. 1H-NMR spectra were recorded on a Bruker Ultra-Shield 400 MHz spectrometer. Samples were dissolved in CDCl3 or D2O as solvent and chemical shifts are reported in ppm. FT-IR spectra were recorded on a Perkin Elmer spectrometer in the range of 4000–400 cm-1 using KBr disk. Mass spectra were obtained using a Kratos MALDI-TOF mass spectrophotometer system. UV-vis absorption spectrum was recorded on a Shimadzu UV-2600 spectrophotometer Photoluminescence spectrum was recorded on JASCO FR 8500 spectrophotometer. TGA was performed a METTLER TOLEDO TGA/851e thermal analyser under nitrogen atmosphere at a heating rate of 10°C min. The liquid crystal properties were determined using differential scanning calorimetry (DSC) from METTLER TOLEDO model DSC822e at range of temperature of 25 to 200°C. Throughout the DSC analysis, the sample was heated and cooled at the rate of 10°C min–1. Polarized optical microscopy (POM) was performed on a Leica DM2700 P model optical polarizing microscope equipped with a Linkam LTS420 hot–stage.
Synthesis of 1,3,5-Triazine-2,4,6-Tricarboxylic acid (2)
Triethyl-1,3,5-triazine-2,4,6-tricarboxylate 1 (1.0 g, 3.36 mmol) was transferred into a 250 mL round bottom flask fitted with a Liebig condenser. Separately, NaOH (0.672 g, 16.8 mmol) was dissolved in 50 mL mixture of water-ethanol (1:1) to form a homogenous solution. The solution was then added dropwise to the triethyl-1,3,5-triazine-2,4,6-tricarboxylate, and the resultant mixture was heated to reflux for 10 hours while stirring. After cooling to room temperature, the pH of the resulting solution was adjusted to pH 2 with 0.1 M HCl after which a white precipitate was obtained. It was then filtered, recrystallized in ice-cold water and dried to give a white powder solid. Yield: 0.7 g, 3.28 mmol, 97%. 1H NMR (400 MHz, CDCl3 δ/ppm): 10.68 (s, 3H, OH). FTIR (KBr): 3447 (m), 1744 (s), 1651 (w), 1416 (m) 1248 (s) 852 cm-1 (m).
Synthesis of 1,3,5-triazine-2,4,6-tricarbonyl trichloride (3)
1,3,5-triazine-tricarboxylic acid 2 (0.5 g, 2.35 mmol) was transferred into a 250 mL two-necked round-bottom flask and was treated under vacuum for 10 minutes before being flowed with nitrogen gas after which 40 mL DMF was added and stirred at 0oC for 1 hour. Then, oxalyl chloride (1 mL, 7.75 mmol) was added dropwise, and the resulting solution was stirred at room temperature overnight. The mixture was then heated for 2 h at 70oC to remove any excess oxalyl chloride before allowing it to cool to room temperature. The solvent was removed by heating at 90oC under reduced pressure and vacuum dried overnight to give a pale pink solid product. The obtained solid was used for further synthesis without purification.
Synthesis of 1,3,5-triazine-2,4,6-tricarboxamide (5)
1,3,4-triazinetricarbonyl trichloride 3 (0.631 g, 2.35 mmol) was placed into a two-neck round bottom flask and was treated under vacuum for 10 minutes before being flowed with nitrogen gas. Then 1-aminododecane (1.44 g, 7.76 mmol) was added dropwise, and the mixture was added with 50 mL dried chloroform, followed by the addition of triethylamine (1.09 mL, 7.76 mmol). After the addition was completed, the reaction mixture was stirred overnight at room temperature, and the resulting mixture was filtered. The targeted product was successfully isolated using column chromatography with chloroform/methanol 50:1 as an eluent and subsequently evaporated and dried under inert condition by using vacuum pump under reduced pressure at room temperature to afford a white solid powder product. Yield: 0.87g, 1.22 mmol, 52 %. 1H NMR (400 MHz, CDCl3 δ/ppm): 0.83-0.87 (t, J = 7.2 Hz, 9H, -CH3), 1.22- 1.35 (overlapped, 54H, -CH2-(CH2)10) 1.70-1.79 (m, 6H -CH2) 2.93-2.97 (t, J = 8.0 Hz, 6H -NHCH2), 8.20(s, 3H, NH). FTIR (KBr): 3430 (M), 1630 (s), 1585 (m), 1516 (w) 1475 (m), 1371 (m), 1143, (s) 835 cm-1(m) MS (MALDI-TOF): m/z 753.5757 [M + K+] observed, calcd for C42H78N6O3: 715.6208 [M + H+].
Results and Discussion
Scheme 1 illustrates the structures and the synthetic route to 1,3,5-triazine-2,4,6-tricarboxamide (5). The target compound was synthesized by Schotten-Baumann amidation.
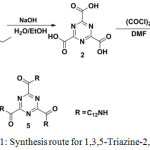 |
Scheme 1: Synthesis route for 1,3,5-Triazine-2,4,6-Tricarboxamide. Click here to View scheme |
Reaction,14 starting with the base hydrolysis of triethyl-1,3,5-triazine-2,4,6-tricarboxylate 1 to afford 1,3,5-triazine-2,4,6-tricarboxylic acid 2 in good yield. Subsequently, compound 2 was subjected to acylation reaction with oxalyl chloride, (COCl)2, in the presence of DMF resulting in the formation of 1,3,4-triazinetricarbonyl trichloride 3 which was then treated with 1-aminododecane in the presence of CHCl3/Et3N to obtain 5 as white powder in 52% yield.
 |
Figure 1: 1H-NMR spectra of (a) 1 and (b) 2. |
Table 1: 1H-NMR data for 1 and 2.
| Proton (H) | Chemical shift (ppm) | |
| 1 | 2 | |
| a | 1.43-1.47 (9H) | − |
| b | 4.52-4.55 (6H) | − |
| c | − | 10.68 (3H) |
Based on the H-NMR spectra in Figure 1 summarized in table 1, the spectrum of precursor 1 dissolved in CDCl3 showed two signals corresponding to the methylene and methyl protons while the product 2 dissolved in D2O showed one signal belong to the hydroxyl proton. The chemical shift at 1.43-1.47 ppm (triplet, 9H, = Ha -CH3) and 4.52-4.57 ppm (quartet, 6H, Hb = -CH2) of compound 1 disappeared in the spectrum of 2 due to cleavage of the ethyl group. Moreover, the appearance of peak at 10.68 ppm (singlet, 3H, Hc = OH) indicate the successful formation of compound 2. It was further confirmed with FTIR as shown in Figure. 2a, by the disappearance of C–H vibrations in the region 3000–2883 cm−1 indicating the cleavage of OCH2CH3 bond in compound 2 and the appearance of broad peak at 3447 cm−1 assigned to O-H stretching vibration of 2 in Figure. 2b.15
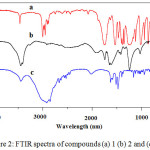 |
Figure 2: FTIR spectra of compounds (a) 1 (b) 2 and (c) 5. |
Figure 3 showed 1HNMR spectra of the reactants 1-aminododecane (4) and product 1,3,5-triazine-2,4,6-tricarboxamide (5) as summarized in table 2. The chemical shift of 1-aminododecane at 2.60-2.65 ppm (t, J = 8.0 Hz, 2H -NHCH2) in Figure. 3a was shifted to 2.93-2.97 (t, J = 8.0 Hz, 6H -NHCH2) in Figure. 3b due to the successful transformation of acyl chloride group of 3 to form carboxamide group. Also the appearances of a broad peak at chemical shift 8.20 ppm (s, 3H, NH) in Figure. 3b indicates the attachment of alkyl side chains to triazine ring. Moreover, the formation of 5 was further confirmed by FTIR spectra in Figure. 2c by the appearance of vibrational peaks at 3436 cm-1 attributed to N-H stretching and 1558 cm-1 N-H bending as well as 2849-2950 cm−1 assigned to C-H stretching and 1475 cm−1 C-H bending, which show that the alkyl side chains was successfully bonded to the triazine ring. It was Further supported by MALDI-TOF-MS molecular weight m/z 753.5757 Da [M+K+] observed, 715.6208 Da [M+H+] calculated for chemical formula C42H78N6O3.
 |
Figure 3: 1H-NMR spectra of (a) 4 and (b) 5. |
Table 2: 1H-NMR data for 4 and 5.
| Proton (H) | Chemical shift (ppm) | |
| 4 | 5 | |
| a | 0.77-0.86 (3H) | 0.83-0.87 (9) |
| b | 1.29-1.41 (20H) | 1.22-135 (54H) |
| c | 2.60-2.65 (2H) | 2.93-2.97 (6H) |
| d | ─ | 1.70-1.79 (6H) |
| e | ─ | 8.20 (3H) |
Photoluminescent Behaviour
1,3,5-triazine-2,4,6-tricarboxamide exhibits fluorescent emission maxima centered at 417 nm and 468 nm on excitation at 254 nm. The luminescent response was investigated in solid and solution. It shows weak fluorescent emission intensity in chloroform solution, in contrast, it gave strong emission intensity in the solid state as shown in Figure. 4a. Upon excitation at 365 nm with hand-held UV lamp, the solid powder gave blue emission, but when the solution of 5 was illuminated at 365 nm, practically no emission was observed Figure 4b. Furthermore, the luminescent behaviour was investigated using UV-Vis absorption in solid state and solution as shown in Figure 4c. In solid state 5 exhibit intense broadened absorption bands from 250-800 nm red shifted with respect to absorption in solution, suggesting intermolecular charge transfer (CT) interactions due to formation of supramolecular J-aggregate. However, in chloroform solution, the broad intense absorption band collapsed suggesting dissolved molecular species and the decreased in intermolecular charge transfer (CT) interactions, which is evident by a blue shift observed and a sharp absorption band at 231 nm suggesting molecular isolated species.
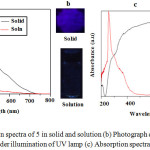 |
Figure 4: (a) Emission spectra of 5 in solid and solution (b) Photograph of its emission in solid and solution under illumination of UV lamp (c) Absorption spectra in solid and solution. |
The intense emission of 5 in solid could be due to aggregation-induced emission (AIE). And its AIE mechanism could be accounted for similar to the previous reports.9,16 The plausible AIE mechanism is shown in figure 5. In contrast to the ACQ effect as a result of free intramolecular rotations along C-C bonds, molecules with AIE properties exhibit strong fluorescent in the aggregate state, attributed to the restriction of intramolecular rotations. It was suggested that multiple non covalent interactions such as hydrogen bonding would stiffen the structure of the molecules upon aggregation hindering free intramolecular rotations.17,18
It is proposed that in the solution state, 5 exist as isolated molecular species which undergo free intramolecular rotations along the amide units upon excitation, which may result in the loss of excitons energy leads to the quenching of the fluorescent emission intensity. However, in the aggregate or solid state, the free intramolecular rotations are restricted due to multiple intermolecular interactions which blocked the non radiative relaxation pathway resulting in the stronger fluorescent emission.
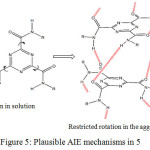 |
Figure 5: Plausible AIE mechanisms in 5. |
Liquid crystal properties
The liquid crystal behaviour was investigated using polarized optical microscopy (POM) and differential scanning calorimetry (DSC). The heating and cooling rate were 10oC/min. Upon cooling from its isotropic phase at 190oC to anisotropic texture the POM showed the appearance of focal conic fan-like texture a typical characteristic of hexagonal columnar mesophase (Colh),19 as shown in Figure. 6a and b. The DSC thermogram displays two endothermic transitions at 73.4oC and 185.6oC upon heating from crystalline (Cr) phase to isotropic (Iso) phase and two exothermic peaks at 181.9oC and 66.1oC when cooled from the isotropic to crystalline phase as shown in Figure. 6c. In addition, two crystalline modifications peaks were observed upon cooling (Cr and Cr1) related to the crystallization process.20 We believed that the transition from the isotropic phase to crystal phase on cooling is accompanied by a reorganization of the molecule in which crystal structural modification is likely to occur.
 |
Figure 6: POM images of 5 upon cooling from isotropic state (a) at 200 µm (b) at 100 µm and (c) DSC thermogram of 5 upon heating and cooling cycle. |
Thermal behaviour
The thermogravimetric analysis (TGA) property of 5 has two identifiable weight loss stages as shown in Figure. 7. It starts in a temperature range of 200°C with a 1% weight loss corresponding to the organic structural collapse of the triazine ring and the alkyl chain. Above 200°C, the compound showed gradual decrease in weight up to 95% and finally decomposed at 330oC. Thus this thermal analysis data suggested that the compound is stable up to 200°C.
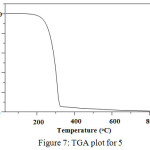 |
Figure 7: TGA plot for 5. |
Conclusions
In summary, we successfully prepared and characterized AIE-active liquid crystal material based on 1,3,5-triazine-2,4,6-tricarboxamide. On the basis of the results obtained, the material was weakly emissive in solution but emit efficiently in the aggregate state, and formed Columnar hexagonal LC phase with focal conic-fan shape texture in addition the molecule exhibit high thermal stability. By combining the advantages of aggregation induce emission and liquid crystal properties such as long-range self-assembly and charge carrier mobility, make this material good candidate for optoelectronic applications.
Acknowledgements
The authors would like to thank Universiti Teknologi Malaysia for the research facilities. AHU highly acknowledged the Tertiary Education Trust Fund (TETFUND) Nigeria and Adamawa State University, Mubi, Nigeria for the financial support.
References
- Gupta, V. K.; Singh, R. A. Aggregation-induced enhanced green light emission from a simple donor–π–acceptor (D–π–A) material: a structure–property relationship study. Faraday discussions, 2017,196, 131-142.
CrossRef - Zhang, J. N.; Kang, H.; Li, N.; Zhou, S. M.; Sun, H. M.; Yin, S. W.; Zhao, N.; Tang, B. Z. Organic solid fluorophores regulated by subtle structure modification: color-tunable and aggregation-induced emission. Chem. Sci., 2017, 8, 577-582.
CrossRef - Carayon, C.; Fery-Forgues, S. 2-Phenylbenzoxazole derivatives: a family of robust emitters of solid-state fluorescence. Photochem. Photobiol. Sci., 2017, 7, 1020-35.
CrossRef - Xue, S.; Qiu, X.; Sun, Q.; Yang, W. Alkyl length effects on solid-state fluorescence and mechanochromic behaviour of small organic luminophores. J. Mater. Chem. C., 2016, 4, 1568-1578.
CrossRef - Yang, J. S.; Yan, J. L. Central-ring functionalization and application of the rigid, aromatic, and H-shaped pentiptycene scaffold. ChemComm., 2008, 13, 1501-12.
CrossRef - Hecht, S.; Fréchet, J. M. Dendritic encapsulation of function: applying nature’s site isolation principle from biomimetics to materials science. Angew. Chem. Int. Ed., 2001, 40, 74-91.
CrossRef - Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. ChemComm., 2001, 18, 1740-1.
CrossRef - Hong Y. Aggregation-induced emission—fluorophores and applications. Methods and applications in fluorescence. 2016, 4(2), 022003.
CrossRef - Jing, H.; Lu, L., Feng, Y.; Zheng, J. F.; Deng, L.; Chen, E. Q.; Ren, X. K. Synthesis, Aggregation-Induced Emission, and Liquid Crystalline Structure of Tetraphenylethylene–Surfactant Complex via Ionic Self-Assembly. J. Phys. Chem. C., 2016, 120(48), 27577-86.
CrossRef - Zhao, D.; Fan, F.; Cheng, J., Zhang, Y.; Wong, K.S.; Chigrinov, VG.; Kwok, H. S.; Guo, L.; Tang, B. Z. Light‐Emitting Liquid Crystal Displays Based on an Aggregation‐Induced Emission Luminogen. Adv. Opt. Mater., 2015, 3(2), 199-202.
CrossRef - Lam, J. W.; Kong, X.; Dong, Y.; Cheuk, K. K.; Xu, K.; Tang, B. Z. Synthesis and properties of liquid crystalline polyacetylenes with different spacer lengths and bridge orientations. Macromolecules. 2000, 14, 5027-40.
CrossRef - Yuan, W. Z.; Yu, Z. Q.; Lu, P.; Deng, C.; Lam, J. W.; Wang Z.; Chen E. Q.; Ma, Y.; Tang, B. Z. High efficiency luminescent liquid crystal: aggregation-induced emission strategy and biaxially oriented mesomorphic structure. J. Mater. Chem., 2012, 8, 3323-6.
CrossRef - Zhao D. Liquid Crystalline AIE Luminogens: Properties and Applications. In: Aggregation-Induced Emission: Materials and Applications Volume 2. Washington: American Chemical Society, 2016, 151-171.
CrossRef - Matmin, J.; Yuliati, L.; Shamsuddin, M.; Lintang, H. O. Supramolecular Hydrogen Bonding Interactions of Novel 1, 3, 5-Benzenetricarbonyl Trisubstituted Alkyl for Anion Sensor Applications. Adv. Mater. Res. 2014, 925, 228-232 Trans Tech Publications.
CrossRef - Wade, Jr.; L. G. Organic Chemistry, 6thd Pearson Education Inc. New Jersey, 2006, 524-525.
- Mei, J.; Hong, Y.; Lam.; J. W.; Qin, A.; Tang, Y.; Tang, B. Z. Aggregation‐induced emission: the whole is more brilliant than the parts. Adv. Mater., 2014, 26(31), 5429-79.
CrossRef - Shyamal, M.; Maity, S.; Mazumdar, P.; Sahoo, G. P.; Maity, R.; Misra, A. Synthesis of an efficient Pyrene based AIE active functional material for selective sensing of 2, 4, 6-trinitrophenol. J. Photochem Photobiol A Chem., 2017, 342, 1-14.
CrossRef - Hong Y.; Jacky W. Y.; Tang, B. Z. Aggregation-induced emission. Chem. Soc. Rev., 2011, 40, 5361-5388
CrossRef - Girotto E.; Eccher J.; Vieira, A. A.; Bechtold, I. H.; Gallardo H. Luminescent columnar liquid crystals based on 1, 3, 4-oxadiazole. Tetrahedron. 2014, 20, 3355-60.
CrossRef - Wróbel, S.; Chruściel, J.; Wierzejska-Adamowicz, M.; Marzec, M.; Ossowska-Chruściel, D. M.; Legrand, C.; Douali, R. Ferroelectric liquid crystals composed of banana-shaped thioesters. In: Ferroelectrics-Physical Effects. MickaA L. Ed., Rijeka: InTechOpen, 2011, 429-448
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









